当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Scalable One-Pot - Liquid-Phase Oligonucleotide Synthesis for Model Network Hydrogels
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2020-09-09 , DOI: 10.1021/jacs.0c05488 Guido Creusen 1, 2, 3 , Cecilia Oluwadunsin Akintayo 1, 2, 3, 4 , Katja Schumann 1 , Andreas Walther 1, 2, 3, 4
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2020-09-09 , DOI: 10.1021/jacs.0c05488 Guido Creusen 1, 2, 3 , Cecilia Oluwadunsin Akintayo 1, 2, 3, 4 , Katja Schumann 1 , Andreas Walther 1, 2, 3, 4
Affiliation
|
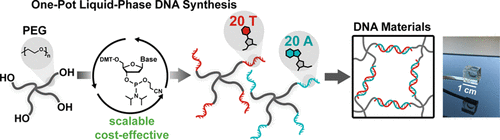
Solid-phase oligonucleotide synthesis (SPOS) based on phosphoramidite chemistry is currently the most widespread technique for DNA and RNA synthesis, but suffers from scalability limitations and high reagent consumption. Liquid-phase oligonucleotide synthesis (LPOS) uses soluble polymer supports and has the potential of being scalable. However, at present, LPOS requires 3 separate reaction steps and 4-5 precipitation steps per nucleotide addition. Moreover, long acid exposure times during the deprotection step degrade sequences with high A-content (adenine) due to depurination and chain cleavage. In this work, we present the first one-pot liquid-phase DNA synthesis technique, which allows the addition of one nucleotide in a one-pot reaction of sequential coupling, oxidation and deprotection, followed by a single precipitation step. Furthermore, we demonstrate how to suppress depurination during the addition of adenine nucleotides. We showcase the potential of this technique to prepare high-purity 4-arm PEG T20 (T = thymine) and 4-arm PEG-A20 building blocks in multi-gram scale. Such complementary 4-arm PEG-DNA building blocks reversibly self-assemble into supramolecular model network hydrogels, and facilitate the elucidation of bond lifetimes. These model network hydrogels exhibit new levels of mechanical properties (storage modulus, bond lifetimes) in DNA bonds at room temperature (melting at 44 °C), and thus open up pathways to next-generation DNA-materials programmable through sequence recognition and available for macroscale applications.
中文翻译:

用于模型网络水凝胶的可扩展一锅法液相寡核苷酸合成
基于亚磷酰胺化学的固相寡核苷酸合成 (SPOS) 是目前最广泛使用的 DNA 和 RNA 合成技术,但存在可扩展性限制和试剂消耗高的问题。液相寡核苷酸合成 (LPOS) 使用可溶性聚合物载体并具有可扩展的潜力。然而,目前,LPOS 每次添加核苷酸需要 3 个单独的反应步骤和 4-5 个沉淀步骤。此外,由于脱嘌呤和链断裂,在去保护步骤中长时间的酸暴露会降解具有高 A 含量(腺嘌呤)的序列。在这项工作中,我们提出了第一个一锅液相 DNA 合成技术,该技术允许在顺序偶联、氧化和去保护的一锅反应中添加一个核苷酸,然后是一个沉淀步骤。此外,我们演示了如何在添加腺嘌呤核苷酸的过程中抑制脱嘌呤。我们展示了这种技术在制备多克规模的高纯度 4 臂 PEG T20(T = 胸腺嘧啶)和 4 臂 PEG-A20 构件方面的潜力。这种互补的 4 臂 PEG-DNA 构建块可逆地自组装成超分子模型网络水凝胶,并有助于阐明键寿命。这些模型网络水凝胶在室温下(44°C 熔化)的 DNA 键中表现出新水平的机械性能(存储模量、键寿命),从而开辟了通过序列识别可编程的下一代 DNA 材料的途径,并且可用用于宏观应用。我们展示了这种技术在制备多克规模的高纯度 4 臂 PEG T20(T = 胸腺嘧啶)和 4 臂 PEG-A20 构件方面的潜力。这种互补的 4 臂 PEG-DNA 构建块可逆地自组装成超分子模型网络水凝胶,并有助于阐明键寿命。这些模型网络水凝胶在室温下(44°C 熔化)的 DNA 键中表现出新水平的机械性能(存储模量、键寿命),从而开辟了通过序列识别可编程的下一代 DNA 材料的途径,并且可用用于宏观应用。我们展示了这种技术在制备多克规模的高纯度 4 臂 PEG T20(T = 胸腺嘧啶)和 4 臂 PEG-A20 构件方面的潜力。这种互补的 4 臂 PEG-DNA 构建块可逆地自组装成超分子模型网络水凝胶,并有助于阐明键寿命。这些模型网络水凝胶在室温下(44°C 熔化)的 DNA 键中表现出新水平的机械性能(存储模量、键寿命),从而开辟了通过序列识别可编程的下一代 DNA 材料的途径,并且可用用于宏观应用。并有助于阐明键寿命。这些模型网络水凝胶在室温下(44°C 熔化)的 DNA 键中表现出新水平的机械性能(存储模量、键寿命),从而开辟了通过序列识别可编程的下一代 DNA 材料的途径,并且可用用于宏观应用。并有助于阐明键寿命。这些模型网络水凝胶在室温下(44°C 熔化)的 DNA 键中表现出新水平的机械性能(存储模量、键寿命),从而开辟了通过序列识别可编程的下一代 DNA 材料的途径,并且可用用于宏观应用。
更新日期:2020-09-09
中文翻译:

用于模型网络水凝胶的可扩展一锅法液相寡核苷酸合成
基于亚磷酰胺化学的固相寡核苷酸合成 (SPOS) 是目前最广泛使用的 DNA 和 RNA 合成技术,但存在可扩展性限制和试剂消耗高的问题。液相寡核苷酸合成 (LPOS) 使用可溶性聚合物载体并具有可扩展的潜力。然而,目前,LPOS 每次添加核苷酸需要 3 个单独的反应步骤和 4-5 个沉淀步骤。此外,由于脱嘌呤和链断裂,在去保护步骤中长时间的酸暴露会降解具有高 A 含量(腺嘌呤)的序列。在这项工作中,我们提出了第一个一锅液相 DNA 合成技术,该技术允许在顺序偶联、氧化和去保护的一锅反应中添加一个核苷酸,然后是一个沉淀步骤。此外,我们演示了如何在添加腺嘌呤核苷酸的过程中抑制脱嘌呤。我们展示了这种技术在制备多克规模的高纯度 4 臂 PEG T20(T = 胸腺嘧啶)和 4 臂 PEG-A20 构件方面的潜力。这种互补的 4 臂 PEG-DNA 构建块可逆地自组装成超分子模型网络水凝胶,并有助于阐明键寿命。这些模型网络水凝胶在室温下(44°C 熔化)的 DNA 键中表现出新水平的机械性能(存储模量、键寿命),从而开辟了通过序列识别可编程的下一代 DNA 材料的途径,并且可用用于宏观应用。我们展示了这种技术在制备多克规模的高纯度 4 臂 PEG T20(T = 胸腺嘧啶)和 4 臂 PEG-A20 构件方面的潜力。这种互补的 4 臂 PEG-DNA 构建块可逆地自组装成超分子模型网络水凝胶,并有助于阐明键寿命。这些模型网络水凝胶在室温下(44°C 熔化)的 DNA 键中表现出新水平的机械性能(存储模量、键寿命),从而开辟了通过序列识别可编程的下一代 DNA 材料的途径,并且可用用于宏观应用。我们展示了这种技术在制备多克规模的高纯度 4 臂 PEG T20(T = 胸腺嘧啶)和 4 臂 PEG-A20 构件方面的潜力。这种互补的 4 臂 PEG-DNA 构建块可逆地自组装成超分子模型网络水凝胶,并有助于阐明键寿命。这些模型网络水凝胶在室温下(44°C 熔化)的 DNA 键中表现出新水平的机械性能(存储模量、键寿命),从而开辟了通过序列识别可编程的下一代 DNA 材料的途径,并且可用用于宏观应用。并有助于阐明键寿命。这些模型网络水凝胶在室温下(44°C 熔化)的 DNA 键中表现出新水平的机械性能(存储模量、键寿命),从而开辟了通过序列识别可编程的下一代 DNA 材料的途径,并且可用用于宏观应用。并有助于阐明键寿命。这些模型网络水凝胶在室温下(44°C 熔化)的 DNA 键中表现出新水平的机械性能(存储模量、键寿命),从而开辟了通过序列识别可编程的下一代 DNA 材料的途径,并且可用用于宏观应用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号