当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
4,4′-Methylene-bis-(2-chloroaniline) Dissolved in Some Neat Solvents: Saturated Solubility, Mixing Properties, and Solvent Effect
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-09-09 , DOI: 10.1021/acs.jced.0c00655 Jian Wang 1 , Xin Yuan 1 , Jiaxin Wu 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-09-09 , DOI: 10.1021/acs.jced.0c00655 Jian Wang 1 , Xin Yuan 1 , Jiaxin Wu 1
Affiliation
|
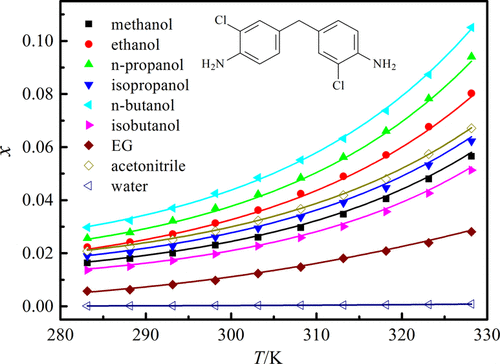
The saturated solubility of 4,4′-methylene-bis-(2-chloroaniline) in some pure solvents involving 1,4-dioxane, methanol, ethanol, N,N-dimethylformamide (DMF), n-propanol, isopropanol, N,N-dimethylacetamide (DMA), n-butanol, water, isobutanol, ethylene glycol (EG), ethyl acetate, and acetonitrile was experimentally achieved at intervals of 5 K via the shake-flask approach at pressure p = 101.2 kPa and temperatures from 283.15 to 328.15 K. Within the temperature range examined, the equilibrium solubility values of 4,4′-methylene-bis-(2-chloroaniline) in the mole fraction increased with an increase in the studied temperature. The solubility magnitudes followed the decreasing trend as: DMA > DMF > 1,4-dioxane > ethyl acetate > n-butanol > n-propanol > ethanol > acetonitrile > isopropanol > methanol > isobutanol > EG > water. X-ray power diffraction analysis displayed that there did not exist crystalline change or solvation in the equilibration process of 4,4′-methylene-bis-(2-chloroaniline) dissolved in the studied neat solvents. The interactions of solvent–solute and solvent–solvent molecules were studied through linear solvation energy relationships to illustrate the solubility variation. The 4,4′-methylene-bis-(2-chloroaniline) solubility was correlated by the Wilson model, Apelblat equation, λh equation, and NRTL model. The obtained maximum values of root-mean-square and relative average deviations were, correspondingly, 11.73 × 10–3 and 4.87%. The Apelblat equation gave better correlation outcomes than the other equations/models. Additionally, the mixing properties were derived from the solubility magnitudes applied to the Wilson model.
中文翻译:

溶解在某些纯溶剂中的4,4'-亚甲基双-(2-氯苯胺):饱和溶解度,混合特性和溶剂效应
4,4'-亚甲基双-(2-氯苯胺)在一些纯溶剂中的饱和溶解度,包括1,4-二恶烷,甲醇,乙醇,N,N-二甲基甲酰胺(DMF),正丙醇,异丙醇,N,通过摇瓶法在压力p下以5 K的间隔实验获得了N-二甲基乙酰胺(DMA),正丁醇,水,异丁醇,乙二醇(EG),乙酸乙酯和乙腈= 101.2 kPa,温度从283.15到328.15K。在所研究的温度范围内,摩尔分数中的4,4'-亚甲基-双-(2-氯苯胺)的平衡溶解度值随着所研究温度的增加而增加。溶解度大小遵循下降趋势:DMA> DMF> 1,4-二恶烷>乙酸乙酯>正丁醇> n丙醇>乙醇>乙腈>异丙醇>甲醇>异丁醇> EG>水。X射线功率衍射分析表明,在纯溶剂中溶解的4,4'-亚甲基双-(2-氯苯胺)的平衡过程中不存在结晶变化或溶剂化。通过线性溶剂化能量关系研究了溶剂-溶质和溶剂-溶剂分子之间的相互作用,以说明溶解度的变化。的4,4'-亚甲基-双- (2-氯苯胺)的溶解度是由Wilson模型,Apelblat方程,λ相关ħ方程,NRTL模型。所获得的均方根最大值和相对平均偏差分别为11.73×10 –3和4.87%。与其他方程式/模型相比,Apelblat方程式具有更好的相关结果。此外,混合特性是从应用于Wilson模型的溶解度得出的。
更新日期:2020-10-08
中文翻译:

溶解在某些纯溶剂中的4,4'-亚甲基双-(2-氯苯胺):饱和溶解度,混合特性和溶剂效应
4,4'-亚甲基双-(2-氯苯胺)在一些纯溶剂中的饱和溶解度,包括1,4-二恶烷,甲醇,乙醇,N,N-二甲基甲酰胺(DMF),正丙醇,异丙醇,N,通过摇瓶法在压力p下以5 K的间隔实验获得了N-二甲基乙酰胺(DMA),正丁醇,水,异丁醇,乙二醇(EG),乙酸乙酯和乙腈= 101.2 kPa,温度从283.15到328.15K。在所研究的温度范围内,摩尔分数中的4,4'-亚甲基-双-(2-氯苯胺)的平衡溶解度值随着所研究温度的增加而增加。溶解度大小遵循下降趋势:DMA> DMF> 1,4-二恶烷>乙酸乙酯>正丁醇> n丙醇>乙醇>乙腈>异丙醇>甲醇>异丁醇> EG>水。X射线功率衍射分析表明,在纯溶剂中溶解的4,4'-亚甲基双-(2-氯苯胺)的平衡过程中不存在结晶变化或溶剂化。通过线性溶剂化能量关系研究了溶剂-溶质和溶剂-溶剂分子之间的相互作用,以说明溶解度的变化。的4,4'-亚甲基-双- (2-氯苯胺)的溶解度是由Wilson模型,Apelblat方程,λ相关ħ方程,NRTL模型。所获得的均方根最大值和相对平均偏差分别为11.73×10 –3和4.87%。与其他方程式/模型相比,Apelblat方程式具有更好的相关结果。此外,混合特性是从应用于Wilson模型的溶解度得出的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号