当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Anomalous Melting Point of Multicharge Ionic Liquids: Structural, Electrostatic, and Orbital Properties of [Ln(NO3)6]3- (Ln = Ce, Pr) Anions.
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2020-09-09 , DOI: 10.1021/acs.inorgchem.0c02078 Wen-Li Yuan 1 , Shuang-Long Wang 1 , You Wang 1 , Lei Zhang 1 , Ling He 1 , Guo-Hong Tao 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2020-09-09 , DOI: 10.1021/acs.inorgchem.0c02078 Wen-Li Yuan 1 , Shuang-Long Wang 1 , You Wang 1 , Lei Zhang 1 , Ling He 1 , Guo-Hong Tao 1
Affiliation
|
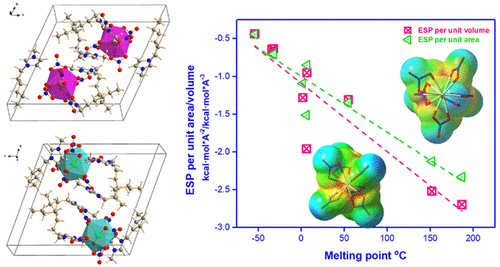
Salts composed of multicharged cations/anions usually exhibit a large lattice energy and strong Coulomb force, which results in high melting points. However, an increasing number of highly charged ionic liquids exceed expectations based on conventional experience; even their melting points are much lower than those found for simple ionic liquids composed of monovalent ions. To further study this phenomenon, we studied a group of stable ionic liquids containing tricharged [Ce(NO3)6]3– and [Pr(NO3)6]3– anions. The structures for [C6mim]3[Ce(NO3)6] and [C6mim]3[Pr(NO3)6] were determined by single-crystal X-ray diffraction with triclinic and P1̅ space groups. The electrostatic potential density per unit ion surface and volume was proposed and calculated. Additionally, theoretical analysis based on Hirshfeld surface and charge decomposition was carried out to explore the intermolecular interaction and electronic structure of the lanthanide anions. The electrostatic and orbital properties were found to be more useful for understanding the melting points of highly charged salts compared with the sole use of lattice energy. The electrostatic potential density per unit ion surface and volume showed a linear relationship with the melting point of ionic liquids composed of monovalent to trivalent ions. These structure–melting point relationships will be beneficial for expounding new low-melting-point ionic liquids with a wide liquidus range.
中文翻译:

多电荷离子液体的异常熔点:[Ln(NO3)6] 3-(Ln = Ce,Pr)阴离子的结构,静电和轨道性质。
由多电荷阳离子/阴离子组成的盐通常显示出大的晶格能和强大的库仑力,从而导致高熔点。但是,越来越多的高电荷离子液体超出了传统经验所期望的水平。甚至它们的熔点也比由一价离子组成的简单离子液体的熔点低得多。为了进一步研究这种现象,我们研究了一组稳定的离子液体,其中含有三电荷的[Ce(NO 3)6 ] 3–和[Pr(NO 3)6 ] 3-阴离子。[C 6 mim] 3 [Ce(NO 3)6 ]和[C 6的结构mim] 3 [Pr(NO 3)6 ]由三斜晶系和P单晶X射线衍射测定1个空间组。提出并计算了单位离子表面和体积的静电势密度。此外,基于Hirshfeld表面和电荷分解进行了理论分析,以探索镧系元素阴离子的分子间相互作用和电子结构。与仅使用晶格能相比,发现静电和轨道性质对于理解高电荷盐的熔点更有用。每单位离子表面和体积的静电势密度与由一价至三价离子组成的离子液体的熔点呈线性关系。这些结构-熔点关系将有助于阐明液相线范围较宽的新型低熔点离子液体。
更新日期:2020-09-21
中文翻译:

多电荷离子液体的异常熔点:[Ln(NO3)6] 3-(Ln = Ce,Pr)阴离子的结构,静电和轨道性质。
由多电荷阳离子/阴离子组成的盐通常显示出大的晶格能和强大的库仑力,从而导致高熔点。但是,越来越多的高电荷离子液体超出了传统经验所期望的水平。甚至它们的熔点也比由一价离子组成的简单离子液体的熔点低得多。为了进一步研究这种现象,我们研究了一组稳定的离子液体,其中含有三电荷的[Ce(NO 3)6 ] 3–和[Pr(NO 3)6 ] 3-阴离子。[C 6 mim] 3 [Ce(NO 3)6 ]和[C 6的结构mim] 3 [Pr(NO 3)6 ]由三斜晶系和P单晶X射线衍射测定1个空间组。提出并计算了单位离子表面和体积的静电势密度。此外,基于Hirshfeld表面和电荷分解进行了理论分析,以探索镧系元素阴离子的分子间相互作用和电子结构。与仅使用晶格能相比,发现静电和轨道性质对于理解高电荷盐的熔点更有用。每单位离子表面和体积的静电势密度与由一价至三价离子组成的离子液体的熔点呈线性关系。这些结构-熔点关系将有助于阐明液相线范围较宽的新型低熔点离子液体。











































 京公网安备 11010802027423号
京公网安备 11010802027423号