当前位置:
X-MOL 学术
›
Energy Fuels
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Lattice Dynamics Study of the Thermal Expansion of C3H8-, CH4-, CF4-, CO2-, Xe-, and N2-Hydrates
Energy & Fuels ( IF 5.2 ) Pub Date : 2020-09-09 , DOI: 10.1021/acs.energyfuels.0c01872 Rodion V. Belosludov 1 , Ravil K. Zhdanov 2, 3 , Yulia Y. Bozhko 2, 3 , Kirill V. Gets 2, 3 , Oleg S. Subbotin 2, 3 , Yoshiyuki Kawazoe 4, 5 , Vladimir R. Belosludov 2, 3
Energy & Fuels ( IF 5.2 ) Pub Date : 2020-09-09 , DOI: 10.1021/acs.energyfuels.0c01872 Rodion V. Belosludov 1 , Ravil K. Zhdanov 2, 3 , Yulia Y. Bozhko 2, 3 , Kirill V. Gets 2, 3 , Oleg S. Subbotin 2, 3 , Yoshiyuki Kawazoe 4, 5 , Vladimir R. Belosludov 2, 3
Affiliation
|
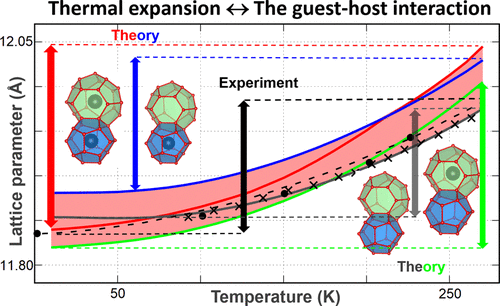
The lattice dynamics method in the quasi-harmonic approximation has been used to study the thermal expansion of C3H8-, CH4-, CF4-, CO2-, Xe-, and N2-hydrates over a wide temperature range. By accounting for the anharmonic nature of interactions inside the hydrate, the model shows good agreement with the reported experimental data. The values of the thermal expansion coefficient for both the empty hydrate and hexagonal ice are smaller than for hydrates with enclathrated guests. The effect of guest size on the lattice parameter of clathrate hydrates in comparison with the lattice of ice Ih has been also investigated. It has been shown that the thermal expansion of the hydrate lattice depends on the cage structure, type and size of the guest, and the cage occupancy, including multiple occupancy. The lattice compression of the hydrate has been found after the inclusion of guest molecules into the large water cavities. In the high-temperature region, the lattice begins to expand relative to the lattice of the empty hydrate. In the case of the hydrate with cubic structure I, the filling of only small cavities results in significant volume expansion relative to the empty hydrate structure over the entire studied temperature range. This confirms the importance of large cage filling for stabilization of clathrate hydrate.
中文翻译:

C 3 H 8-,CH 4-,CF 4-,CO 2-,Xe-和N 2-水合物热膨胀的晶格动力学研究
准谐波近似中的晶格动力学方法已用于研究C 3 H 8-,CH 4-,CF 4-,CO 2-,Xe-和N 2-水合物在较宽温度范围内的热膨胀。通过考虑水合物内部相互作用的非调和性质,该模型显示出与报道的实验数据良好的一致性。空水合物和六角形冰的热膨胀系数值均小于具有包封客体的水合物的热膨胀系数值。与冰晶格I h相比,客体大小对笼形水合物水合物晶格参数的影响也已被调查。已经显示出水合物晶格的热膨胀取决于笼结构,客体的类型和大小以及笼的占有率,包括多个占有率。在将客体分子包含到大的水腔中之后,发现了水合物的晶格压缩。在高温区域,晶格开始相对于空水合物的晶格扩展。在水合物具有立方结构I的情况下,在整个研究温度范围内,相对于空的水合物结构,仅小空腔的填充会导致明显的体积膨胀。这证实了大笼子装填对于稳定笼形水合物的重要性。
更新日期:2020-10-16
中文翻译:

C 3 H 8-,CH 4-,CF 4-,CO 2-,Xe-和N 2-水合物热膨胀的晶格动力学研究
准谐波近似中的晶格动力学方法已用于研究C 3 H 8-,CH 4-,CF 4-,CO 2-,Xe-和N 2-水合物在较宽温度范围内的热膨胀。通过考虑水合物内部相互作用的非调和性质,该模型显示出与报道的实验数据良好的一致性。空水合物和六角形冰的热膨胀系数值均小于具有包封客体的水合物的热膨胀系数值。与冰晶格I h相比,客体大小对笼形水合物水合物晶格参数的影响也已被调查。已经显示出水合物晶格的热膨胀取决于笼结构,客体的类型和大小以及笼的占有率,包括多个占有率。在将客体分子包含到大的水腔中之后,发现了水合物的晶格压缩。在高温区域,晶格开始相对于空水合物的晶格扩展。在水合物具有立方结构I的情况下,在整个研究温度范围内,相对于空的水合物结构,仅小空腔的填充会导致明显的体积膨胀。这证实了大笼子装填对于稳定笼形水合物的重要性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号