当前位置:
X-MOL 学术
›
J. Phys. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanisms of Csp3‐H or Nsp2‐H functionalization of 2,3‐diaminoindoles with triplet O2: A density functional theory investigation
Journal of Physical Organic Chemistry ( IF 1.9 ) Pub Date : 2020-09-09 , DOI: 10.1002/poc.4134 Zhe‐Peng Deng 1 , Da‐Gang Zhou 2
Journal of Physical Organic Chemistry ( IF 1.9 ) Pub Date : 2020-09-09 , DOI: 10.1002/poc.4134 Zhe‐Peng Deng 1 , Da‐Gang Zhou 2
Affiliation
|
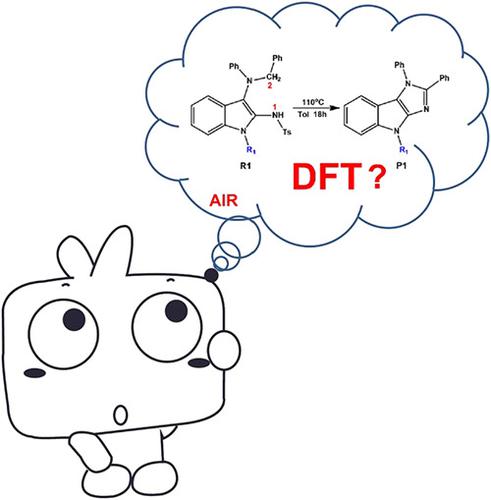
The mechanisms of Csp3‐H or Nsp2‐H activations of 2,3‐diaminoindoles oxidized by triplet O2 have been investigated with the method of M06‐2X‐D3/6‐31 + G(d,p) in the Universal Solvation Model Based on Solute Electron Density (SMD) model. The calculations indicate that the activation of 2,3‐diaminoindoles has three possible paths. First, the Csp3‐H functionalization has a higher energy barrier than that of Nsp2‐H activation; then an intramolecular H‐shift reaction happened to lose a H2O2 molecule; the third step is a ring‐closure reaction and imidazolo‐indole skeleton would be constructed; finally, the elimination reaction occurred, and the final product would be yielded. Fukui function and dual descriptor could be used to predict the active sites of the reactants and intermediates. Results would provide valuable insights into these types of interactions and related ones.
中文翻译:

2,3-二氨基吲哚与三重态O2的Csp3-H或Nsp2-H功能化的机理:密度泛函理论研究
用通用的M06-2X-D3 / 6-31 + G(d,p)方法研究了被三重态O 2氧化的2,3-二氨基吲哚的Csp 3 -H或Nsp 2 -H活化机理基于溶质电子密度(SMD)模型的溶质模型。计算表明2,3-二氨基吲哚的活化具有三种可能的途径。首先,Csp 3 -H功能化的能垒比Nsp 2 -H激活的能垒高。分子内的H位移反应恰好失去了H 2 O 2分子; 第三步是闭环反应,并构建咪唑并吲哚骨架。最后,发生消除反应,得到最终产物。Fukui函数和双重描述符可用于预测反应物和中间体的活性位点。结果将提供对这些类型的交互和相关交互的有价值的见解。
更新日期:2020-09-09
中文翻译:

2,3-二氨基吲哚与三重态O2的Csp3-H或Nsp2-H功能化的机理:密度泛函理论研究
用通用的M06-2X-D3 / 6-31 + G(d,p)方法研究了被三重态O 2氧化的2,3-二氨基吲哚的Csp 3 -H或Nsp 2 -H活化机理基于溶质电子密度(SMD)模型的溶质模型。计算表明2,3-二氨基吲哚的活化具有三种可能的途径。首先,Csp 3 -H功能化的能垒比Nsp 2 -H激活的能垒高。分子内的H位移反应恰好失去了H 2 O 2分子; 第三步是闭环反应,并构建咪唑并吲哚骨架。最后,发生消除反应,得到最终产物。Fukui函数和双重描述符可用于预测反应物和中间体的活性位点。结果将提供对这些类型的交互和相关交互的有价值的见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号