当前位置:
X-MOL 学术
›
J. Neurochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Examination of genetic and pharmacological tools to study the proteasomal deubiquitinating enzyme ubiquitin‐specific protease 14 in the nervous system
Journal of Neurochemistry ( IF 4.7 ) Pub Date : 2020-09-09 , DOI: 10.1111/jnc.15180 Tina Tian 1 , John W McLean 1 , Julie A Wilson 1 , Scott M Wilson 1
Journal of Neurochemistry ( IF 4.7 ) Pub Date : 2020-09-09 , DOI: 10.1111/jnc.15180 Tina Tian 1 , John W McLean 1 , Julie A Wilson 1 , Scott M Wilson 1
Affiliation
|
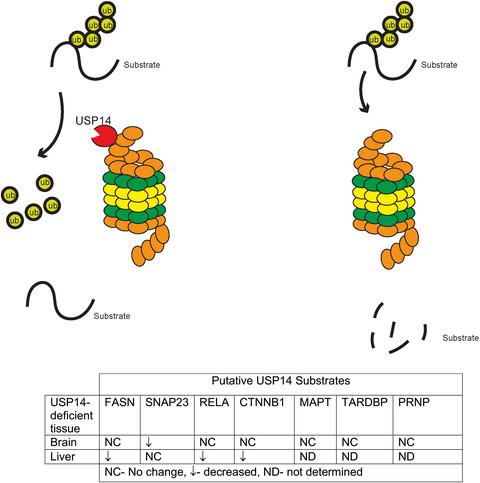
Strategies for enhancing protein degradation have been proposed for treating neurological diseases associated with a decline in proteasome activity. A proteasomal deubiquitinating enzyme that controls substrate entry into proteasomes, ubiquitin‐specific protease 14 (USP14), is an attractive candidate for therapies that modulate proteasome activity. This report tests the validity of genetic and pharmacological tools to study USP14’s role in regulating protein abundance. Although previous studies implicated USP14 in the degradation of microtubule associate protein tau, tar DNA binding protein, and prion protein, the levels of these proteins were similar in our neurons cultured from wild type and USP14‐deficient mice. Neither loss nor over‐expression of USP14 affected the levels of these proteins in mice, implying that modifying the amount of USP14 is not sufficient to alter their steady‐state levels. However, neuronal over‐expression of a catalytic mutant of USP14 showed that manipulating USP14’s ubiquitin‐hydrolase activity altered the levels of specific proteins in vivo. Although pharmacological inhibitors of USP14’s ubiquitin‐hydrolase activity reduced microtubule associate protein tau, tar DNA binding protein, and prion protein in culture, the effect was similar in wild type and USP14‐deficient neurons, thus impacting their use for specifically evaluating USP14 in a therapeutic manner. While examining how targeting USP14 may affect other proteins in vivo, this report showed that fatty acid synthase, v‐rel reticuloendotheliosis viral oncogene homolog, CTNNB1, and synaptosome associated protein 23 are reduced in USP14‐deficient mice; however, loss of USP14 differentially altered the levels of these proteins in the liver and brain. As such, it is critical to more thoroughly examine how inhibiting USP14 alters protein abundance to determine if targeting USP14 will be a beneficial strategy for treating neurodegenerative diseases.
中文翻译:

研究神经系统中蛋白酶体去泛素化酶泛素特异性蛋白酶 14 的遗传和药理学工具
已经提出了用于治疗与蛋白酶体活性下降相关的神经系统疾病的增强蛋白质降解的策略。一种控制底物进入蛋白酶体的蛋白酶体去泛素化酶,泛素特异性蛋白酶 14 (USP14),是调节蛋白酶体活性疗法的有吸引力的候选者。本报告测试了研究 USP14 在调节蛋白质丰度中的作用的遗传和药理学工具的有效性。尽管之前的研究表明 USP14 与微管相关蛋白 tau、焦油 DNA 结合蛋白和朊病毒蛋白的降解有关,但这些蛋白质的水平在我们从野生型和 USP14 缺陷小鼠培养的神经元中相似。USP14 的缺失或过度表达均不影响小鼠中这些蛋白质的水平,这意味着修改 USP14 的量不足以改变它们的稳态水平。然而,USP14 催化突变体的神经元过度表达表明,操纵 USP14 的泛素水解酶活性改变了体内特定蛋白质的水平。虽然 USP14 泛素水解酶活性的药理学抑制剂降低了微管相关蛋白 tau、焦油 DNA 结合蛋白和朊病毒蛋白,但在野生型和 USP14 缺陷神经元中的效果相似,因此影响了它们在治疗中特异性评估 USP14 的用途方式。在检查靶向 USP14 可能如何影响体内其他蛋白质时,该报告显示脂肪酸合酶、v-rel 网状内皮增生病毒癌基因同源物、CTNNB1 和突触体相关蛋白 23 在 USP14 缺陷小鼠中减少;然而,USP14 的缺失会不同程度地改变这些蛋白质在肝脏和大脑中的水平。因此,更彻底地检查抑制 USP14 如何改变蛋白质丰度以确定靶向 USP14 是否是治疗神经退行性疾病的有益策略至关重要。
更新日期:2020-09-09
中文翻译:

研究神经系统中蛋白酶体去泛素化酶泛素特异性蛋白酶 14 的遗传和药理学工具
已经提出了用于治疗与蛋白酶体活性下降相关的神经系统疾病的增强蛋白质降解的策略。一种控制底物进入蛋白酶体的蛋白酶体去泛素化酶,泛素特异性蛋白酶 14 (USP14),是调节蛋白酶体活性疗法的有吸引力的候选者。本报告测试了研究 USP14 在调节蛋白质丰度中的作用的遗传和药理学工具的有效性。尽管之前的研究表明 USP14 与微管相关蛋白 tau、焦油 DNA 结合蛋白和朊病毒蛋白的降解有关,但这些蛋白质的水平在我们从野生型和 USP14 缺陷小鼠培养的神经元中相似。USP14 的缺失或过度表达均不影响小鼠中这些蛋白质的水平,这意味着修改 USP14 的量不足以改变它们的稳态水平。然而,USP14 催化突变体的神经元过度表达表明,操纵 USP14 的泛素水解酶活性改变了体内特定蛋白质的水平。虽然 USP14 泛素水解酶活性的药理学抑制剂降低了微管相关蛋白 tau、焦油 DNA 结合蛋白和朊病毒蛋白,但在野生型和 USP14 缺陷神经元中的效果相似,因此影响了它们在治疗中特异性评估 USP14 的用途方式。在检查靶向 USP14 可能如何影响体内其他蛋白质时,该报告显示脂肪酸合酶、v-rel 网状内皮增生病毒癌基因同源物、CTNNB1 和突触体相关蛋白 23 在 USP14 缺陷小鼠中减少;然而,USP14 的缺失会不同程度地改变这些蛋白质在肝脏和大脑中的水平。因此,更彻底地检查抑制 USP14 如何改变蛋白质丰度以确定靶向 USP14 是否是治疗神经退行性疾病的有益策略至关重要。



























 京公网安备 11010802027423号
京公网安备 11010802027423号