当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Forming All-Carbon Quaternary Stereocenters by Organocatalytic Aminomethylation: Concise Access to β2,2-Amino Acids.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-09-09 , DOI: 10.1002/anie.202009892 Kai Wang 1 , Jianliang Yu 1 , Ying Shao 1 , Shengbiao Tang 1 , Jiangtao Sun 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-09-09 , DOI: 10.1002/anie.202009892 Kai Wang 1 , Jianliang Yu 1 , Ying Shao 1 , Shengbiao Tang 1 , Jiangtao Sun 1
Affiliation
|
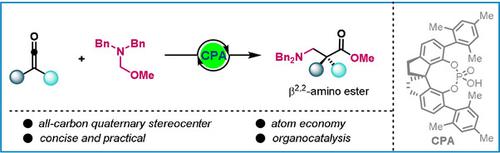
The asymmetric synthesis of β2,2‐amino acids remains a formidable challenge in organic synthesis. Here a novel organocatalytic enantioselective aminomethylation of ketenes with stable and readily available N,O‐acetals is reported, providing β2,2‐amino esters bearing an all‐carbon quaternary stereogenic center in high enantiomeric ratios with a catalytic amount of chiral phosphoric acid. Typically, this transformation probably proceeds through an asymmetric counter‐anion‐directed catalysis. As a result, a concise, practical, and atom‐economic protocol toward rapidly access to β2,2‐amino acids has been developed.
中文翻译:

通过有机催化的氨甲基化形成全碳四元立体中心:精确获得β2,2-氨基酸。
的β不对称合成2,2- α-氨基酸保留在有机合成一个巨大的挑战。这里有稳定且容易获得的N,O-缩醛烯酮的一种新颖的对映选择性有机催化氨基甲基报道,提供β 2,2-氨基酯轴承在高对映体比率的全碳季立体中心具有手性磷酸催化量。通常,这种转变可能是通过不对称的反阴离子导向催化进行的。其结果是,一个简洁,实用,和原子的经济朝向快速访问β协议2,2 α-氨基酸已经研制成功。
更新日期:2020-09-09
中文翻译:

通过有机催化的氨甲基化形成全碳四元立体中心:精确获得β2,2-氨基酸。
的β不对称合成2,2- α-氨基酸保留在有机合成一个巨大的挑战。这里有稳定且容易获得的N,O-缩醛烯酮的一种新颖的对映选择性有机催化氨基甲基报道,提供β 2,2-氨基酯轴承在高对映体比率的全碳季立体中心具有手性磷酸催化量。通常,这种转变可能是通过不对称的反阴离子导向催化进行的。其结果是,一个简洁,实用,和原子的经济朝向快速访问β协议2,2 α-氨基酸已经研制成功。











































 京公网安备 11010802027423号
京公网安备 11010802027423号