Molecular Cell ( IF 14.5 ) Pub Date : 2020-09-09 , DOI: 10.1016/j.molcel.2020.08.012 Melanie A McDowell 1 , Michael Heimes 1 , Francesco Fiorentino 2 , Shahid Mehmood 2 , Ákos Farkas 3 , Javier Coy-Vergara 3 , Di Wu 2 , Jani Reddy Bolla 2 , Volker Schmid 3 , Roger Heinze 1 , Klemens Wild 1 , Dirk Flemming 1 , Stefan Pfeffer 4 , Blanche Schwappach 3 , Carol V Robinson 2 , Irmgard Sinning 1
|
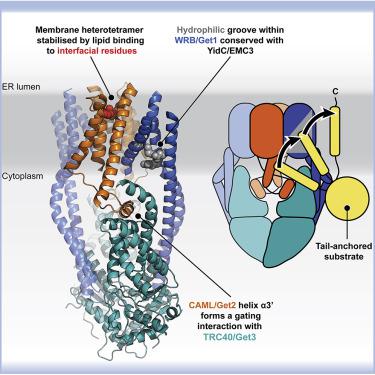
Membrane protein biogenesis faces the challenge of chaperoning hydrophobic transmembrane helices for faithful membrane insertion. The guided entry of tail-anchored proteins (GET) pathway targets and inserts tail-anchored (TA) proteins into the endoplasmic reticulum (ER) membrane with an insertase (yeast Get1/Get2 or mammalian WRB/CAML) that captures the TA from a cytoplasmic chaperone (Get3 or TRC40, respectively). Here, we present cryo-electron microscopy reconstructions, native mass spectrometry, and structure-based mutagenesis of human WRB/CAML/TRC40 and yeast Get1/Get2/Get3 complexes. Get3 binding to the membrane insertase supports heterotetramer formation, and phosphatidylinositol binding at the heterotetramer interface stabilizes the insertase for efficient TA insertion in vivo. We identify a Get2/CAML cytoplasmic helix that forms a “gating” interaction with Get3/TRC40 important for TA insertion. Structural homology with YidC and the ER membrane protein complex (EMC) implicates an evolutionarily conserved insertion mechanism for divergent substrates utilizing a hydrophilic groove. Thus, we provide a detailed structural and mechanistic framework to understand TA membrane insertion.
中文翻译:

通过 GET 插入酶复合物进行尾锚定膜蛋白生物发生的结构基础。
膜蛋白生物发生面临着陪伴疏水性跨膜螺旋以实现忠实膜插入的挑战。尾锚定蛋白 (GET) 通路的引导进入靶向尾锚定 (TA) 蛋白并将其插入内质网 (ER) 膜,并使用插入酶(酵母 Get1/Get2 或哺乳动物 WRB/CAML)捕获 TA 从细胞质伴侣(分别为 Get3 或 TRC40)。在这里,我们展示了人类 WRB/CAML/TRC40 和酵母 Get1/Get2/Get3 复合物的冷冻电子显微镜重建、原生质谱和基于结构的诱变。Get3 与膜插入酶的结合支持异源四聚体的形成,而异源四聚体界面处的磷脂酰肌醇结合稳定了插入酶以实现体内有效的 TA 插入. 我们确定了一个 Get2/CAML 细胞质螺旋,它与 Get3/TRC40 形成“门控”相互作用,对于 TA 插入很重要。与 YidC 和 ER 膜蛋白复合物 (EMC) 的结构同源性暗示了利用亲水凹槽的发散底物的进化上保守的插入机制。因此,我们提供了一个详细的结构和机械框架来理解 TA 膜插入。











































 京公网安备 11010802027423号
京公网安备 11010802027423号