Biomaterials Advances ( IF 5.5 ) Pub Date : 2020-09-09 , DOI: 10.1016/j.msec.2020.111498 Nasrin Zohreh , Zahra Rastegaran , Seyed Hassan Hosseini , Mehdi Akhlaghi , Cosmin Istrate , Cristina Busuioc
|
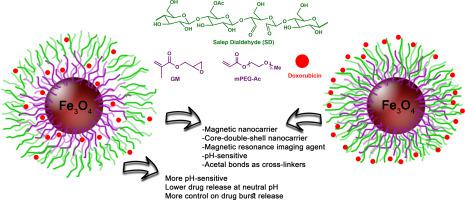
Two core-double-shell pH-sensitive nanocarriers were fabricated using Fe3O4 as magnetic core, poly(glycidyl methacrylate-PEG) and salep dialdehyde as the first and the second shell, and doxorubicin as the hydrophobic anticancer drug. Two nanocarriers were different in the drug loading steps. The interaction between the first and the second shell assumed to be pH-sensitive via acetal cross linkages. The structure of nanocarriers, organic shell loading, magnetic responsibility, morphology, size, dispersibility, and drug loading content were investigated by IR, NMR, TG, VSM, XRD, DLS, HRTEM and UV-Vis analyses. The long-term drug release profiles of both nanocarriers showed that the drug loading before cross-linking between the first and second shell led to a more pH-sensitive nanocarrier exhibiting higher control on DOX release. Cellular toxicity assay (MTT) showed that DOX-free nanocarrier is biocompatible having cell viability greater than 80% for HEK-293 and MCF-7 cell lines. Besides, high cytotoxic effect observed for drug-loaded nanocarrier on MCF-7 cancer cells. Cellular uptake analysis showed that the nanocarrier is able to transport DOX into the cytoplasm and perinuclear regions of MCF-7 cells. In vitro hemolysis and coagulation assays demonstrated high blood compatibility of nanocarrier. The results also suggested that low concentration of nanocarrier have a great potential as a contrast agent in magnetic resonance imaging (MRI).
中文翻译:

基于聚甲基丙烯酸缩水甘油酯的双壳磁性纳米载体的pH引发阿霉素的细胞内释放
使用Fe 3 O 4制备了两种核-双壳pH敏感纳米载体作为磁芯,聚(甲基丙烯酸缩水甘油酯-PEG)和沙利普二醛作为第一和第二壳层,阿霉素作为疏水性抗癌药物。两种纳米载体的载药步骤不同。假设第一和第二壳之间的相互作用通过缩醛交联对pH敏感。通过IR,NMR,TG,VSM,XRD,DLS,HRTEM和UV-Vis分析,研究了纳米载体的结构,有机壳载量,磁性,形貌,尺寸,分散性和载药量。两种纳米载体的长期药物释放曲线表明,第一壳和第二壳之间交联之前的载药量导致了对pH敏感度更高的纳米载体,对DOX的释放表现出更高的控制力。细胞毒性试验(MTT)表明,不含DOX的纳米载体对HEK-293和MCF-7细胞系具有超过80%的细胞活力,具有生物相容性。此外,载药纳米载体对MCF-7癌细胞具有高的细胞毒性作用。细胞摄取分析表明,纳米载体能够将DOX转运到MCF-7细胞的细胞质和核周区域。体外溶血和凝血测定表明纳米载体具有高度的血液相容性。结果还表明,低浓度的纳米载体在磁共振成像(MRI)中具有作为造影剂的巨大潜力。细胞摄取分析表明,纳米载体能够将DOX转运到MCF-7细胞的细胞质和核周区域。体外溶血和凝血测定表明纳米载体具有高度的血液相容性。结果还表明,低浓度的纳米载体在磁共振成像(MRI)中具有作为造影剂的巨大潜力。细胞摄取分析表明,纳米载体能够将DOX转运到MCF-7细胞的细胞质和核周区域。体外溶血和凝血测定表明纳米载体具有高度的血液相容性。结果还表明,低浓度的纳米载体在磁共振成像(MRI)中具有作为造影剂的巨大潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号