Dyes and Pigments ( IF 4.1 ) Pub Date : 2020-09-09 , DOI: 10.1016/j.dyepig.2020.108836 Alexander K. Eltyshev , Timur H. Dzhumaniyazov , Polina O. Suntsova , Artem S. Minin , Varvara A. Pozdina , Wim Dehaen , Enrico Benassi , Nataliya P. Belskaya
|
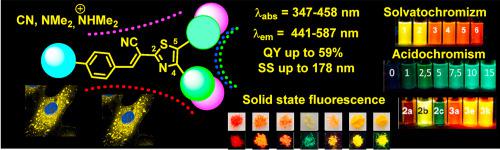
New fluorescent thiazoles were designed and synthesized based on a 3-aryl-2-(thiazol-2-yl)acrylonitrile core. Three synthetic approaches were developed to introduce specific combinations of substituents at the 2-, 4- and 5-thiazole positions. The obtained thiazolyl-2-acrylonitriles exhibited a wide range of fluorescent colours (from green to red), long wavelength maxima and intensity depending on the combination of the substituents located at rings A, B and C. The expanded photophysical investigation established the best substituent combinations to increase their emission. Absorption and emission were studied in solvents with different polarities, as well as in DMSO-water and dioxane-water mixtures. The thiazoles showed multifunctional properties and exhibited good emission in the solid phase and in suspension (aggregation induced enhancement emission/AIEE effect). Photophysical investigations revealed a large Stokes shift, significant positive solvatochromism, and the tunability of the colour and intensity. Sharp strengthening of the emission intensity of the thiazoles was observed upon stimulation with some acid (H2SO4 and BF3·OEt2) in solvents and in the solid phase (HCl). State-of-the-art quantum mechanical calculations were performed to interpret the experimental findings. Biological experiments revealed the good penetration of the thiazoles into living cells and the accumulation both in lysosomes and, to a lesser extent, near membranes.
中文翻译:

芳基/杂芳基环组装的3-芳基-2-(噻唑-2-基)丙烯腈:光学性质设计和应用前景
基于3-芳基-2-(噻唑-2-基)丙烯腈核设计并合成了新型荧光噻唑。开发了三种合成方法以在2-,4-和5-噻唑位置引入取代基的特定组合。所获得的噻唑基-2-丙烯腈表现出宽范围的荧光色(从绿色到红色),长波长最大值和强度,取决于位于环A,B和C的取代基的组合。扩展的光物理研究确定了最佳的取代基组合以增加其排放。在不同极性的溶剂以及DMSO-水和二恶烷-水的混合物中研究了吸收和发射。噻唑显示出多功能特性,并在固相和悬浮液中表现出良好的发射(聚集诱导增强发射/ AIEE效应)。光物理研究表明,斯托克斯频移很大,明显的溶剂化变色现象明显,并且颜色和强度可调。在某些酸刺激下,噻唑的发射强度急剧增强(H2 SO 4和BF 3 ·OEt 2)在溶剂中和在固相(HCl)中。进行了最新的量子力学计算以解释实验结果。生物实验表明,噻唑能够很好地渗透到活细胞中,并且在溶酶体中和(在较小程度上)在膜附近都有积累。











































 京公网安备 11010802027423号
京公网安备 11010802027423号