Chemical Physics ( IF 2.0 ) Pub Date : 2020-09-09 , DOI: 10.1016/j.chemphys.2020.110975 A.L. Shimkevich
|
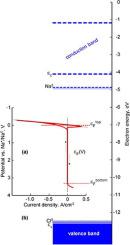
A band gap of the molten sodium chloride is estimated more than 7 eV but its electrochemical window is less than 4 eV. These parameters limit a true mole solubility of metal sodium in its molten chloride by ~10−7% mol at 1173 K that is much less than 3% mol equal to the observed solubility of this metal as neutral atoms in the homogeneous molten sodium chloride at the same temperature. Such great difference between these magnitudes and transparency of the liquid with the observed sodium solubility indicate the existence of a micro-emulsion in the system, Na–NaCl when the high observed solubility of metal sodium in its molten chloride is a result of mixing two phases in the form of ultra thin dispersion of liquid metal and molten salt having the eutectic point Na0.06(NaCl)0.94.
中文翻译:

与观察到的金属钠在熔融氯化物中的溶解度相比,这是真正的溶解度
熔融氯化钠的带隙估计大于7 eV,但其电化学窗口小于4 eV。这些参数在1173 K时将金属钠在其熔融氯化物中的真实摩尔溶解度限制为〜10 -7%mol,远小于3%mol,这等于该金属在中性熔融氯化钠中在中性原子中所观察到的溶解度相同的温度。这些量值与观察到的钠溶解度的液体透明度之间的如此巨大差异表明,当观察到金属钠在其熔融氯化物中的高溶解度时,系统中存在一种微乳液Na-NaCl。以液态金属和熔融盐的超薄分散体形式存在,其共晶点为Na 0.06(NaCl)0.94。











































 京公网安备 11010802027423号
京公网安备 11010802027423号