Applied Catalysis A: General ( IF 4.7 ) Pub Date : 2020-09-09 , DOI: 10.1016/j.apcata.2020.117824 Brandie L. Rhodes , John D. DeSain , Paul D. Ronney
|
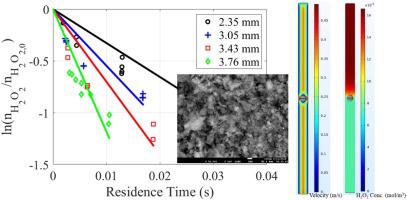
An experimental and numerical study of the rates of decomposition of hydrogen peroxide vapor on catalytic surfaces was conducted. Hydrogen peroxide vapor and helium carrier gas flowed over a catalyst (platinum on alumina spheres of varying diameter) at a total pressure of <3 Torr, simulating the conditions of a flash evaporation propulsion system for space applications. After reacting on the catalyst, the product gas composition was measured using near-infrared laser absorption on a known hydrogen peroxide line in a multi-pass flow cell. Global reaction rate constants were inferred for varying catalyst surface areas, residence times, and temperatures. The combination of these experimental data with numerical simulations led to the conclusion that under the conditions employed in this study, namely low peroxide partial pressures and low Reynolds numbers, decomposition rates are transport-limited rather than reaction-limited. These results may be applicable to the development of catalyst bed designs for small satellite propulsion systems.
中文翻译:

过氧化氢蒸气与氧化铝球上的铂反应
对过氧化氢蒸气在催化表面上的分解速率进行了实验和数值研究。过氧化氢蒸气和氦气载气以小于3托的总压力流经催化剂(直径可变的氧化铝球体上的铂),模拟了空间应用中闪蒸推进系统的条件。在催化剂上反应后,在多通道流通池中使用已知的过氧化氢管线上的近红外激光吸收来测量产物气体的组成。根据变化的催化剂表面积,停留时间和温度推断出总反应速率常数。这些实验数据与数值模拟相结合得出的结论是,在本研究采用的条件下,即低的过氧化物分压和低的雷诺数,分解速率受运输限制而不是反应受限制。这些结果可能适用于小型卫星推进系统催化剂床设计的开发。











































 京公网安备 11010802027423号
京公网安备 11010802027423号