当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Diastereoselective synthesis of dispiro[indoline-3,3′-furan-2′,3′′-pyrrolidine] via [3 + 2]cycloaddition reaction of MBH maleimides of isatins and 1,3-dicarbonyl compounds
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2020-09-08 , DOI: 10.1039/d0qo00845a Liu-Na Pan 1, 2, 3, 4 , Jing Sun 1, 2, 3, 4 , Rong-Guo Shi 1, 2, 3, 4 , Chao-Guo Yan 1, 2, 3, 4
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2020-09-08 , DOI: 10.1039/d0qo00845a Liu-Na Pan 1, 2, 3, 4 , Jing Sun 1, 2, 3, 4 , Rong-Guo Shi 1, 2, 3, 4 , Chao-Guo Yan 1, 2, 3, 4
Affiliation
|
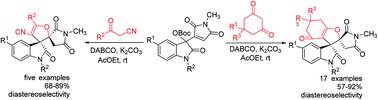
In the presence of mixed bases DABCO and K2CO3, the reaction of MBH maleimides of isatins with various cyclic 1,3-dicarbonyl compounds such as 1,3-cyclohexanedione, dimedone, 4-hydroxychromenone and benzoylacetonitrile afforded functionalized dispiro[indoline-3,3′-furan-2′,3′′-pyrrolidines] in satisfactory yields and with high diastereoselectivity. This formal [3 + 2]cycloaddition proceeded with nucleophilic substitution of an allylammonium ylide and oxa-Michael addition of an enolate. However, a similar reaction of MBH adducts of isatins derived from acrylonitrile and methyl acrylate resulted in functionalized spiro[indoline-3,4′-pyranes] in good yields through formal [3 + 3]cycloaddition reaction.
中文翻译:

通过靛红与1,3-二羰基化合物的MBH马来酰亚胺的[3 + 2]环加成反应,非对映选择性合成二螺[吲哚啉-3,3'-呋喃-2',3''-吡咯烷]
在混合碱DABCO和K 2 CO 3的存在下,靛红的MBH马来酰亚胺与各种环状1,3-二羰基化合物(例如1,3-环己二酮,二甲酮,4-羟基色酮和苯甲酰基乙腈)的反应提供了功能化的双螺[吲哚3,3′-呋喃-2′,3′′-吡咯烷]以令人满意的产率和高非对映选择性。正式的[3 + 2]环加成反应是对烯丙基乙胺盐进行亲核取代,对烯醇盐进行oxa-Michael加成。然而,通过正式的[3 + 3]环加成反应,衍生自丙烯腈和丙烯酸甲酯的isatins的MBH加合物的类似反应导致官能化的螺[吲哚啉-3,4'-吡喃]收率很高。
更新日期:2020-10-13
中文翻译:

通过靛红与1,3-二羰基化合物的MBH马来酰亚胺的[3 + 2]环加成反应,非对映选择性合成二螺[吲哚啉-3,3'-呋喃-2',3''-吡咯烷]
在混合碱DABCO和K 2 CO 3的存在下,靛红的MBH马来酰亚胺与各种环状1,3-二羰基化合物(例如1,3-环己二酮,二甲酮,4-羟基色酮和苯甲酰基乙腈)的反应提供了功能化的双螺[吲哚3,3′-呋喃-2′,3′′-吡咯烷]以令人满意的产率和高非对映选择性。正式的[3 + 2]环加成反应是对烯丙基乙胺盐进行亲核取代,对烯醇盐进行oxa-Michael加成。然而,通过正式的[3 + 3]环加成反应,衍生自丙烯腈和丙烯酸甲酯的isatins的MBH加合物的类似反应导致官能化的螺[吲哚啉-3,4'-吡喃]收率很高。











































 京公网安备 11010802027423号
京公网安备 11010802027423号