当前位置:
X-MOL 学术
›
RSC Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design and synthesis of substituted (1-(benzyl)-1H-1,2,3-triazol-4-yl)(piperazin-1-yl)methanone conjugates: study on their apoptosis inducing ability and tubulin polymerization inhibition
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2020-09-08 , DOI: 10.1039/d0md00162g Kesari Lakshmi Manasa 1, 2 , Sowjanya Thatikonda 3 , Dilep Kumar Sigalapalli 1, 2 , Sowmya Vuppaladadium 4 , Ganthala Parimala Devi 1 , Chandraiah Godugu 3 , Mallika Alvala 2 , Narayana Nagesh 4 , Bathini Nagendra Babu 1
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2020-09-08 , DOI: 10.1039/d0md00162g Kesari Lakshmi Manasa 1, 2 , Sowjanya Thatikonda 3 , Dilep Kumar Sigalapalli 1, 2 , Sowmya Vuppaladadium 4 , Ganthala Parimala Devi 1 , Chandraiah Godugu 3 , Mallika Alvala 2 , Narayana Nagesh 4 , Bathini Nagendra Babu 1
Affiliation
|
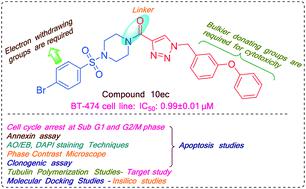
A library of substituted (1-(benzyl)-1H-1,2,3-triazol-4-yl)(piperazin-1-yl)methanone derivatives were designed, synthesized and screened for their in vitro cytotoxic activity against BT-474, HeLa, MCF-7, NCI-H460 and HaCaT cells by employing 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Among all the synthesized analogues, compound 10ec displayed the highest cytotoxicity with the IC50 value of 0.99 ± 0.01 μM towards BT-474 cancer cell line. The target compound (10ec) was also evaluated for its tubulin polymerization inhibition study. Detailed biological studies such as acridine orange/ethidium bromide (AO/EB), DAPI and annexin V-FITC/propidium iodide staining assay suggested that compound 10ec induced the apoptosis of BT-474 cells. The clonogenic assay revealed that the inhibition of colony formation in BT-474 cells by 10ec in concentration-dependent manner. Moreover, the flow cytometric analysis revealed that 10ec induced apoptosis via cell cycle arrest at the sub-G1 and G2/M phase. In silico studies of sulfonyl piperazine-integrated triazole conjugates unveil that they possess drug-like properties. According to the molecular modelling studies, compound 10ec binds to the colchicine binding site of the tubulin.
中文翻译:

取代的(1-(苄基)-1H-1,2,3-三唑-4-基)(哌嗪-1-基)甲酮缀合物的设计与合成:研究其细胞凋亡诱导能力和微管蛋白聚合抑制作用
设计、合成了取代的(1-(苄基)-1 H -1,2,3-三唑-4-基)(哌嗪-1-基)甲酮衍生物文库,并筛选了其针对 BT- 的体外细胞毒活性。采用 3-(4,5-二甲基噻唑-2-基)-2,5-二苯基溴化四唑 (MTT) 测定法检测 474、HeLa、MCF-7、NCI-H460 和 HaCaT 细胞。在所有合成的类似物中,化合物10ec对BT-474癌细胞系表现出最高的细胞毒性,IC 50值为0.99 ± 0.01 μM。还评估了目标化合物 ( 10ec ) 的微管蛋白聚合抑制研究。吖啶橙/溴化乙锭 (AO/EB)、DAPI 和膜联蛋白 V-FITC/碘化丙啶染色测定等详细生物学研究表明,化合物10ec诱导 BT-474 细胞凋亡。克隆形成实验表明, 10ec对 BT-474 细胞中集落形成的抑制作用呈浓度依赖性。此外,流式细胞术分析表明, 10ec通过将细胞周期阻滞在亚 G1 和 G2/M 期来诱导细胞凋亡。对磺酰哌嗪整合的三唑缀合物的计算机研究表明它们具有类似药物的特性。根据分子模型研究,化合物10ec与微管蛋白的秋水仙碱结合位点结合。
更新日期:2020-11-03
中文翻译:

取代的(1-(苄基)-1H-1,2,3-三唑-4-基)(哌嗪-1-基)甲酮缀合物的设计与合成:研究其细胞凋亡诱导能力和微管蛋白聚合抑制作用
设计、合成了取代的(1-(苄基)-1 H -1,2,3-三唑-4-基)(哌嗪-1-基)甲酮衍生物文库,并筛选了其针对 BT- 的体外细胞毒活性。采用 3-(4,5-二甲基噻唑-2-基)-2,5-二苯基溴化四唑 (MTT) 测定法检测 474、HeLa、MCF-7、NCI-H460 和 HaCaT 细胞。在所有合成的类似物中,化合物10ec对BT-474癌细胞系表现出最高的细胞毒性,IC 50值为0.99 ± 0.01 μM。还评估了目标化合物 ( 10ec ) 的微管蛋白聚合抑制研究。吖啶橙/溴化乙锭 (AO/EB)、DAPI 和膜联蛋白 V-FITC/碘化丙啶染色测定等详细生物学研究表明,化合物10ec诱导 BT-474 细胞凋亡。克隆形成实验表明, 10ec对 BT-474 细胞中集落形成的抑制作用呈浓度依赖性。此外,流式细胞术分析表明, 10ec通过将细胞周期阻滞在亚 G1 和 G2/M 期来诱导细胞凋亡。对磺酰哌嗪整合的三唑缀合物的计算机研究表明它们具有类似药物的特性。根据分子模型研究,化合物10ec与微管蛋白的秋水仙碱结合位点结合。











































 京公网安备 11010802027423号
京公网安备 11010802027423号