当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of 3-Deoxy-d-manno-oct-2-ulosonic Acid (KDO) and Pseudaminic Acid C-Glycosides.
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-09-08 , DOI: 10.1021/acs.joc.0c01838 Emmanuel Onobun 1, 2, 3 , David Crich 1, 2, 3
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-09-08 , DOI: 10.1021/acs.joc.0c01838 Emmanuel Onobun 1, 2, 3 , David Crich 1, 2, 3
Affiliation
|
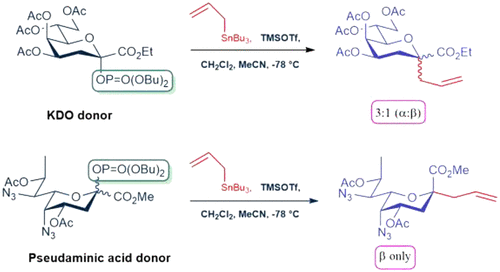
The preparation of glycosyl dibutyl phosphates in the 3-deoxy-d-manno-oct-2-ulosonic acid (KDO) and pseudaminic acid series and their application to the formation of C-glycosides are described. Both donors were obtained from the corresponding thioglycosides by treatment with dibutylphosphoric acid and N-iodosuccinimide. As with the thioglycosides, both donors adopted very predominantly the strongly electron-withdrawing tg conformation of their side chains, which is reflected in the excellent equatorial selectivity of both donors in the formation of exemplary O-glycosides. With respect to C-glycoside formation on the other hand, contrasting results were observed: the KDO donor was either relatively unselective or selective for the formation of the axial C-glycoside, while the pseudaminic acid donor was selective for the formation of the equatorial C-glycoside. These observations are rationalized in terms of the greater electron-withdrawing ability of the azides in the pseudaminic acid donor compared to the corresponding acetoxy groups in the KDO series, resulting in a reaction through tighter ion pairs even at the SN1 end of the general glycosylation mechanism. The contrast in the axial versus the equatorial selectivity between C- and O-glycosylation cautions against the extrapolation of models for SN1-type glycosylation with weak nucleophiles for the explanation of O-glycosylation.
中文翻译:

3-Deoxy-d-manno-oct-2-ulosonic Acid (KDO) 和 Pseudaminic Acid C-Glycosides 的合成。
的糖基二磷酸酯在3脱氧制备d -甘露-辛-2-糖酸(KDO)和pseudaminic酸系列以及它们的形成应用Ç -glycosides中描述。通过用二丁基磷酸和N-碘代琥珀酰亚胺处理,从相应的硫代糖苷获得两个供体。与硫代糖苷一样,两个供体都非常主要地采用其侧链的强吸电子tg构象,这反映在两个供体在示例性O-糖苷形成中的极好的赤道选择性上。关于C在另一方面,观察到对比结果:KDO供体对轴向C-糖苷的形成相对无选择性或选择性,而伪胺酸供体对赤道C-糖苷的形成具有选择性。与 KDO 系列中相应的乙酰氧基相比,伪胺酸供体中的叠氮化物具有更大的吸电子能力,从而使这些观察结果合理化,即使在一般的 S N 1 末端,也能通过更紧密的离子对进行反应。糖基化机制。C - 和O之间轴向与赤道选择性的对比-糖基化警告不要将具有弱亲核试剂的S N 1 型糖基化模型外推来解释O-糖基化。
更新日期:2020-09-08
中文翻译:

3-Deoxy-d-manno-oct-2-ulosonic Acid (KDO) 和 Pseudaminic Acid C-Glycosides 的合成。
的糖基二磷酸酯在3脱氧制备d -甘露-辛-2-糖酸(KDO)和pseudaminic酸系列以及它们的形成应用Ç -glycosides中描述。通过用二丁基磷酸和N-碘代琥珀酰亚胺处理,从相应的硫代糖苷获得两个供体。与硫代糖苷一样,两个供体都非常主要地采用其侧链的强吸电子tg构象,这反映在两个供体在示例性O-糖苷形成中的极好的赤道选择性上。关于C在另一方面,观察到对比结果:KDO供体对轴向C-糖苷的形成相对无选择性或选择性,而伪胺酸供体对赤道C-糖苷的形成具有选择性。与 KDO 系列中相应的乙酰氧基相比,伪胺酸供体中的叠氮化物具有更大的吸电子能力,从而使这些观察结果合理化,即使在一般的 S N 1 末端,也能通过更紧密的离子对进行反应。糖基化机制。C - 和O之间轴向与赤道选择性的对比-糖基化警告不要将具有弱亲核试剂的S N 1 型糖基化模型外推来解释O-糖基化。









































 京公网安备 11010802027423号
京公网安备 11010802027423号