当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Naphthyl-Derived Orthometalated RuII-NHC Complexes: Effect of the NHC Donors and/or Substitution Pattern on Their Synthesis and Catalytic Activity
Organometallics ( IF 2.8 ) Pub Date : 2020-09-07 , DOI: 10.1021/acs.organomet.0c00441 Somnath Bauri 1 , Anirban Mallik 1 , Arnab Rit 1
Organometallics ( IF 2.8 ) Pub Date : 2020-09-07 , DOI: 10.1021/acs.organomet.0c00441 Somnath Bauri 1 , Anirban Mallik 1 , Arnab Rit 1
Affiliation
|
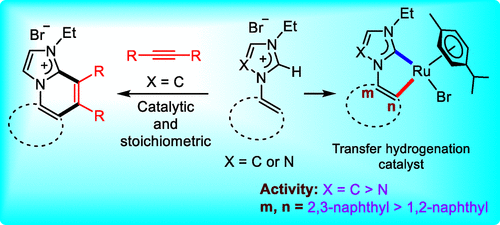
Naphthyl-derived orthometalated RuII-NHC complexes have been developed for catalytic applications considering the stereoelectronic profiles of the NHC ligands, which can be modified easily via alteration of the NHC donors (ImNHC/1,2,4-tzNHC) as well as their substitution pattern at the naphthyl ring. The azolium salts [(2a–c)-H]Br, precursors for the NHC ligands, react with [Ru(p-cymene)Cl2]2 in the presence of a base to deliver the ortho-metalated RuII-NHC complexes 3a–c with the general formula [(NHC)Ru(p-cymene)Br]. Orthometalation in these complexes can be exploited for further functionalization of the NHC ligands. This is depicted by the generation of the diphenylethylene-inserted isolable intermediate complex 4, from the reaction of 3a with diphenylacetylene in a 1:1 ratio, which eventually provides an annulated salt 5a-Br via reductive elimination. Gratifyingly, this process can also be made catalytic, which directly provides several mono-/bis-annulated cationic N-heterocyclic compounds starting from the imidazolium salts [(2a,b)-H]Br using [Ru(p-cymene)Cl2]2 as a precatalyst. Furthermore, subtle variations in the electronic profiles of the complexes 3a–c in combination with steric alterations are observed to influence their activity in the transfer hydrogenation of acetophenone, a model for the hydride transfer process. Among all the complexes studied here, complex 3b with an ImNHC donor at the second position of the naphthyl ring was identified as a superior catalyst in comparison to 3a,c featuring either a different NHC donor or substitution pattern with a low loading of 0.1 mol%.
中文翻译:

萘衍生的正金属Ru II -NHC配合物:NHC供体和/或取代方式对其合成和催化活性的影响
考虑到NHC配体的立体电子特征,已开发出萘基正金属化Ru II -NHC配合物,可通过改变NHC供体(ImNHC / 1,2,4-tzNHC)及其它们萘环上的取代模式。NHC配体的前体偶氮盐[(2a – c)-H] Br与[Ru(p- Cymene)Cl 2 ] 2在碱存在下反应,以提供邻位金属化的Ru II -NHC配合物图3a - ç与通式[(NHC)钌(p-cymene)Br]。这些复合物中的正金属化可用于NHC配体的进一步功能化。这是由3a与二苯基乙炔以1:1的比例反应生成的,插入二苯乙烯的可分离中间体配合物4生成的,该化合物最终通过还原消除反应提供了环化盐5a-Br。令人欣慰的是,该方法也可以催化使用,直接使用[Ru(p- Cymene)Cl 2 ]从咪唑鎓盐[(2a,b)-H] Br开始提供几种单/双环阳离子N-杂环化合物] 2作为前催化剂。此外,观察到配合物3a - c电子构型的细微变化与空间变化共同影响其在苯乙酮(氢化物转移过程的模型)的转移氢化中的活性。在本文研究的所有配合物中,与3a,c相比,在萘环第二位具有ImNHC供体的配合物3b被认为是一种优异的催化剂,其特征在于不同的NHC供体或具有0.1 mol%低载量的取代模式。
更新日期:2020-09-28
中文翻译:

萘衍生的正金属Ru II -NHC配合物:NHC供体和/或取代方式对其合成和催化活性的影响
考虑到NHC配体的立体电子特征,已开发出萘基正金属化Ru II -NHC配合物,可通过改变NHC供体(ImNHC / 1,2,4-tzNHC)及其它们萘环上的取代模式。NHC配体的前体偶氮盐[(2a – c)-H] Br与[Ru(p- Cymene)Cl 2 ] 2在碱存在下反应,以提供邻位金属化的Ru II -NHC配合物图3a - ç与通式[(NHC)钌(p-cymene)Br]。这些复合物中的正金属化可用于NHC配体的进一步功能化。这是由3a与二苯基乙炔以1:1的比例反应生成的,插入二苯乙烯的可分离中间体配合物4生成的,该化合物最终通过还原消除反应提供了环化盐5a-Br。令人欣慰的是,该方法也可以催化使用,直接使用[Ru(p- Cymene)Cl 2 ]从咪唑鎓盐[(2a,b)-H] Br开始提供几种单/双环阳离子N-杂环化合物] 2作为前催化剂。此外,观察到配合物3a - c电子构型的细微变化与空间变化共同影响其在苯乙酮(氢化物转移过程的模型)的转移氢化中的活性。在本文研究的所有配合物中,与3a,c相比,在萘环第二位具有ImNHC供体的配合物3b被认为是一种优异的催化剂,其特征在于不同的NHC供体或具有0.1 mol%低载量的取代模式。



























 京公网安备 11010802027423号
京公网安备 11010802027423号