当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric Synthesis of γ-Secondary Amino Alcohols via a Borrowing-Hydrogen Cascade.
Organic Letters ( IF 4.9 ) Pub Date : 2020-09-08 , DOI: 10.1021/acs.orglett.0c02614 Yupeng Pan 1 , Yipeng You 1 , Dongxu He 1 , Fumin Chen 1 , Xiaoyong Chang 1 , Ming Yu Jin 1 , Xiangyou Xing 1
Organic Letters ( IF 4.9 ) Pub Date : 2020-09-08 , DOI: 10.1021/acs.orglett.0c02614 Yupeng Pan 1 , Yipeng You 1 , Dongxu He 1 , Fumin Chen 1 , Xiaoyong Chang 1 , Ming Yu Jin 1 , Xiangyou Xing 1
Affiliation
|
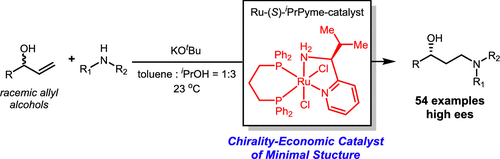
The borrowing-hydrogen (or hydrogen autotransfer) process, where the catalyst dehydrogenates a substrate and formally transfers the H atom to an unsaturated intermediate, is an atom-efficient and environmentally benign transformation. Described here is an example of an asymmetric borrowing-hydrogen cascade for the formal anti-Markovnikov hydroamination of allyl alcohols to synthesize optically enriched γ-secondary amino alcohols. By exploiting the Ru-(S)-iPrPyme catalyst with minimal stereogenicity, a cascade process including dehydrogenation, conjugate addition, and asymmetric reduction was developed. The mild conditions, functional group tolerance, and broad substrate scope (54 examples) demonstrate the synthetic practicality of the catalytic system.
中文翻译:

通过借位氢级联反应不对称合成γ-仲氨基醇。
借贷氢(或氢自动转移)工艺是一种对原子有效的,对环境无害的转化工艺,其中催化剂将底物脱氢并将H原子正式转移至不饱和中间体。这里描述的是不对称的借位氢级联的实例,用于烯丙醇的形式上的反马尔科夫尼科夫加氢胺化反应,以合成光学富集的γ-仲氨基醇。通过利用具有最小立体异构性的Ru- (S)-i PrPyme催化剂,开发了一种包括脱氢,共轭加成和不对称还原的级联过程。温和的条件,官能团的耐受性和广泛的底物范围(54个实例)证明了催化体系的合成实用性。
更新日期:2020-09-20
中文翻译:

通过借位氢级联反应不对称合成γ-仲氨基醇。
借贷氢(或氢自动转移)工艺是一种对原子有效的,对环境无害的转化工艺,其中催化剂将底物脱氢并将H原子正式转移至不饱和中间体。这里描述的是不对称的借位氢级联的实例,用于烯丙醇的形式上的反马尔科夫尼科夫加氢胺化反应,以合成光学富集的γ-仲氨基醇。通过利用具有最小立体异构性的Ru- (S)-i PrPyme催化剂,开发了一种包括脱氢,共轭加成和不对称还原的级联过程。温和的条件,官能团的耐受性和广泛的底物范围(54个实例)证明了催化体系的合成实用性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号