当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Iron-Catalyzed Redox-Neutral Radical Cascade Reaction of [60]Fullerene with γ,δ-Unsaturated Oxime Esters: Preparation of Free (N-H) Pyrrolidino[2',3':1,2]fullerenes.
Organic Letters ( IF 4.9 ) Pub Date : 2020-09-08 , DOI: 10.1021/acs.orglett.0c02658 Conghui Wu 1 , Tong-Xin Liu 1 , Pengling Zhang 1 , Xue Zhu 1 , Guisheng Zhang 1
Organic Letters ( IF 4.9 ) Pub Date : 2020-09-08 , DOI: 10.1021/acs.orglett.0c02658 Conghui Wu 1 , Tong-Xin Liu 1 , Pengling Zhang 1 , Xue Zhu 1 , Guisheng Zhang 1
Affiliation
|
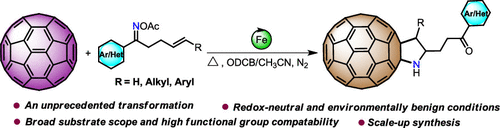
Herein an unprecedented iron(II)-catalyzed redox-neutral radical cascade reaction of [60]fullerene with γ,δ-unsaturated oxime esters is reported for the preparation of novel free (N–H) pyrrolidino[2′,3′:1,2]fullerenes. The transformation undergoes an intramolecular cyclization/intermolecular cyclization/oxidation/hydrolysis cascade, and features simple operation, broad substrate scope/high functional group compatibility as well as suitable for scale-up synthesis, providing a facile and practical access to a range of free pyrrolidino[2′,3′:1,2]fullerenes.
中文翻译:

铁催化[60]富勒烯与γ,δ-不饱和肟酯的氧化还原-中性自由基级联反应:制备游离(NH)吡咯烷基[2',3':1,2]富勒烯。
本文报道了空前的铁(II)催化的[60]富勒烯与γ,δ-不饱和肟酯的铁(II)催化的还原-中性自由基级联反应,用于制备新型的游离(NH)吡咯烷基[2',3':1 ,2]富勒烯。该转化过程经历了分子内环化/分子间环化/氧化/水解的级联反应,并且具有操作简单,底物范围广/官能团相容性高以及适用于按比例放大合成的特点,可轻松,实用地获得各种游离吡咯烷基[2',3':1,2]富勒烯。
更新日期:2020-09-20
中文翻译:

铁催化[60]富勒烯与γ,δ-不饱和肟酯的氧化还原-中性自由基级联反应:制备游离(NH)吡咯烷基[2',3':1,2]富勒烯。
本文报道了空前的铁(II)催化的[60]富勒烯与γ,δ-不饱和肟酯的铁(II)催化的还原-中性自由基级联反应,用于制备新型的游离(NH)吡咯烷基[2',3':1 ,2]富勒烯。该转化过程经历了分子内环化/分子间环化/氧化/水解的级联反应,并且具有操作简单,底物范围广/官能团相容性高以及适用于按比例放大合成的特点,可轻松,实用地获得各种游离吡咯烷基[2',3':1,2]富勒烯。











































 京公网安备 11010802027423号
京公网安备 11010802027423号