当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Palladium-Catalyzed [3+2] Cycloaddition via Two-Fold 1,3-C(sp3)–H Activation
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-09-08 , DOI: 10.1021/jacs.0c08290 Hojoon Park 1 , Jin-Quan Yu 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-09-08 , DOI: 10.1021/jacs.0c08290 Hojoon Park 1 , Jin-Quan Yu 1
Affiliation
|
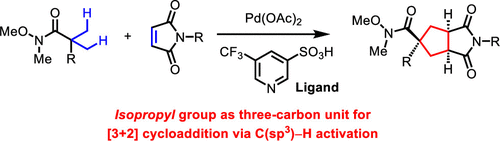
Cycloaddition reactions provide an expeditious route to con-struct ring systems in a highly convergent and stereoselective manner. For a typical cycloaddition reaction to occur, however, the installation of multiple reactive functional groups (π-bonds, leaving group, etc.) are required within the substrates, compro-mising the overall efficiency or scope of the cycloaddition re-action. Here, we report a palladium-catalyzed [3+2] reaction that utilizes two-fold C(sp3)-H activation to generate the three-carbon unit for formal cycloaddition. The initial -C(sp3)-H activation of aliphatic amide/maleimide insertion triggers a re-layed, second C(sp3)-H activation to complete a formal [3+2] cycloaddition. The key to success was the use of weakly coor-dinating amide as the directing group, as undesired Heck or alkylation pathways have been reported with stronger-coordinating directing groups. The use of pyridine-3-sulfonic acid ligands is also crucial to promote the amide-directed C(sp3)-H activation step. This method is compatible with a wide range of amide substrates, including lactams, which lead to spiro-bicyclic products. The [3+2] product is also shown to undergo a reductive desymmetrization process to access chiral cyclopentane bearing multiple stereocenters with excellent en-antioselectivity.
中文翻译:

钯催化的 [3+2] 环加成反应通过二重 1,3-C(sp3)–H 活化
环加成反应提供了一种以高度收敛和立体选择性方式构建环系统的快速途径。然而,要发生典型的环加成反应,需要在底物中安装多个反应性官能团(π 键、离去基团等),这会影响环加成反应的整体效率或范围。在这里,我们报告了钯催化的 [3+2] 反应,该反应利用两倍 C(sp3)-H 活化生成用于正式环加成的三碳单元。脂肪族酰胺/马来酰亚胺插入的初始 -C(sp3)-H 激活触发重新铺设的第二次 C(sp3)-H 激活以完成正式的 [3+2] 环加成。成功的关键是使用弱配位酰胺作为导向基团,已经报道了具有更强配位导向基团的不希望的 Heck 或烷基化途径。吡啶-3-磺酸配体的使用对于促进酰胺导向的 C(sp3)-H 活化步骤也至关重要。该方法与多种酰胺底物兼容,包括内酰胺,可产生螺双环产物。[3+2] 产物还显示出经过还原去对称化过程,以获取具有多个立体中心的手性环戊烷,并具有出色的对映选择性。
更新日期:2020-09-08
中文翻译:

钯催化的 [3+2] 环加成反应通过二重 1,3-C(sp3)–H 活化
环加成反应提供了一种以高度收敛和立体选择性方式构建环系统的快速途径。然而,要发生典型的环加成反应,需要在底物中安装多个反应性官能团(π 键、离去基团等),这会影响环加成反应的整体效率或范围。在这里,我们报告了钯催化的 [3+2] 反应,该反应利用两倍 C(sp3)-H 活化生成用于正式环加成的三碳单元。脂肪族酰胺/马来酰亚胺插入的初始 -C(sp3)-H 激活触发重新铺设的第二次 C(sp3)-H 激活以完成正式的 [3+2] 环加成。成功的关键是使用弱配位酰胺作为导向基团,已经报道了具有更强配位导向基团的不希望的 Heck 或烷基化途径。吡啶-3-磺酸配体的使用对于促进酰胺导向的 C(sp3)-H 活化步骤也至关重要。该方法与多种酰胺底物兼容,包括内酰胺,可产生螺双环产物。[3+2] 产物还显示出经过还原去对称化过程,以获取具有多个立体中心的手性环戊烷,并具有出色的对映选择性。










































 京公网安备 11010802027423号
京公网安备 11010802027423号