当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Simultaneous Control of Central and Helical Chiralities: Expedient Helicoselective Synthesis of Dioxa[6]helicenes
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-09-08 , DOI: 10.1021/jacs.0c07995 Peng Liu 1 , Xiaoze Bao 2 , Jean-Valère Naubron 3 , Sara Chentouf 3 , Stéphane Humbel 1 , Nicolas Vanthuyne 1 , Marion Jean 1 , Laurent Giordano 1 , Jean Rodriguez 1 , Damien Bonne 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-09-08 , DOI: 10.1021/jacs.0c07995 Peng Liu 1 , Xiaoze Bao 2 , Jean-Valère Naubron 3 , Sara Chentouf 3 , Stéphane Humbel 1 , Nicolas Vanthuyne 1 , Marion Jean 1 , Laurent Giordano 1 , Jean Rodriguez 1 , Damien Bonne 1
Affiliation
|
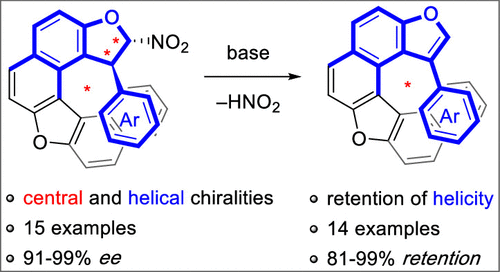
An expedient synthesis of a new family of configurationally stable dioxa[6]helicenes was established using a sequential helicoselective organocatalyzed heteroannulation/eliminative aromatization via enantioenriched fused 2-nitro dihydrofurans featuring both central and helical chiralities. Starting from simple achiral precursors, a broad range of these previously unknown chiral heterocyclic scaffolds were obtained with good efficiency, and their aromatization proceeded with very high enantiopurity retention in most cases.
中文翻译:

中心和螺旋手性的同时控制:二氧杂环己烷[6]螺旋烯的便利螺旋选择性合成
使用顺序螺旋选择性有机催化杂环化/消除芳构化,通过具有中心和螺旋手性的对映体富集的稠合 2-硝基二氢呋喃,建立了构型稳定的二氧杂环 [6] 螺旋新家族的便利合成。从简单的非手性前体开始,以良好的效率获得了范围广泛的这些先前未知的手性杂环支架,并且在大多数情况下它们的芳构化以非常高的对映纯度保留进行。
更新日期:2020-09-08
中文翻译:

中心和螺旋手性的同时控制:二氧杂环己烷[6]螺旋烯的便利螺旋选择性合成
使用顺序螺旋选择性有机催化杂环化/消除芳构化,通过具有中心和螺旋手性的对映体富集的稠合 2-硝基二氢呋喃,建立了构型稳定的二氧杂环 [6] 螺旋新家族的便利合成。从简单的非手性前体开始,以良好的效率获得了范围广泛的这些先前未知的手性杂环支架,并且在大多数情况下它们的芳构化以非常高的对映纯度保留进行。











































 京公网安备 11010802027423号
京公网安备 11010802027423号