当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reversible Hydrogenation–Dehydrogenation of Acetylpyridine-Pd-MIL-101(Cr) for Chemical Hydrogen Storage
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2020-09-08 , DOI: 10.1021/acs.iecr.0c02764 Nuttapong Makmeesub, Chonlada Ritvirulh, Kittisak Choojun, Teng-Hao Chen, Yingyot Poo-arporn, Daniel E. Resasco, Tawan Sooknoi
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2020-09-08 , DOI: 10.1021/acs.iecr.0c02764 Nuttapong Makmeesub, Chonlada Ritvirulh, Kittisak Choojun, Teng-Hao Chen, Yingyot Poo-arporn, Daniel E. Resasco, Tawan Sooknoi
|
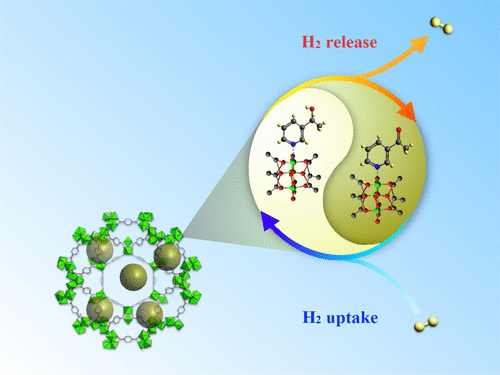
3-Acetylpyridine (AcP), as an organic hydrogen carrier, and Pd nanoparticles, as a catalyst, were incorporated into MIL-101(Cr) for chemical hydrogen storage. AcP was first grafted into MIL-101(Cr), and then Pd (0.5–4.0 wt %) was encapsulated by a double-solvent adsorption process. Thermogravimetric analysis, inductively coupled plasma–optical emission spectrometry, X-ray photoelectron spectroscopy, transmission electron microscopy, in situ X-ray adsorption near-edge structure analysis, 1H nuclear magnetic resonance (NMR), and elemental analysis suggested the existence of AcP and Pd nanoparticles (NPs) inside the MIL-101(Cr) cages. The chemical hydrogen storage of samples was evaluated by H2 temperature-programmed reaction. In situ Fourier transform infrared and 1H NMR techniques verified the hydrogenated and dehydrogenated forms of AcP upon hydrogen uptake. Reversible hydrogenation/dehydrogenation can be readily regulated by H2 partial pressure and temperature. The chemical hydrogen storage could be accomplished only when AcP and Pd NPs were adjacently present. The chemical hydrogen storage was enhanced with an increased Pd loading up to 0.33 mmol H2·g–1 per cycle. With the manipulation of hydrogenation and dehydrogenation temperatures at 150 °C, the chemical hydrogen storage can be maintained for up to 10 cycles. The material reported herein is one of the noncryogenic chemical hydrogen storages that can be operated at constant temperature and atmospheric pressure.
中文翻译:

乙酰吡啶-Pd-MIL-101(Cr)的可逆加氢-脱氢,用于化学储氢
将3-乙酰基吡啶(AcP)(作为有机氢载体)和Pd纳米颗粒(作为催化剂)掺入MIL-101(Cr)中进行化学氢存储。首先将AcP接枝到MIL-101(Cr)中,然后通过双溶剂吸附工艺将Pd(0.5-4.0 wt%)封装。热重分析,电感耦合等离子体发射光谱,X射线光电子能谱,透射电子显微镜,原位X射线吸附近边缘结构分析,1 H核磁共振(NMR)和元素分析表明存在AcP和MIL-101(Cr)笼内的Pd纳米颗粒(NPs)。通过H 2程序升温反应评估样品的化学氢存储量。原位傅立叶变换红外和11 H NMR技术证实了吸氢后AcP的氢化和脱氢形式。H 2的分压和温度可以容易地调节可逆的氢化/脱氢。仅当AcP和Pd NP相邻存在时,才能完成化学氢存储。每个循环中Pd的负载量增加至0.33 mmol H 2 ·g –1时,化学氢存储量得到了增强。通过控制150°C的氢化和脱氢温度,化学氢的存储量最多可维持10个循环。本文报道的材料是可在恒定温度和大气压下运行的非低温化学氢存储装置之一。
更新日期:2020-10-07
中文翻译:

乙酰吡啶-Pd-MIL-101(Cr)的可逆加氢-脱氢,用于化学储氢
将3-乙酰基吡啶(AcP)(作为有机氢载体)和Pd纳米颗粒(作为催化剂)掺入MIL-101(Cr)中进行化学氢存储。首先将AcP接枝到MIL-101(Cr)中,然后通过双溶剂吸附工艺将Pd(0.5-4.0 wt%)封装。热重分析,电感耦合等离子体发射光谱,X射线光电子能谱,透射电子显微镜,原位X射线吸附近边缘结构分析,1 H核磁共振(NMR)和元素分析表明存在AcP和MIL-101(Cr)笼内的Pd纳米颗粒(NPs)。通过H 2程序升温反应评估样品的化学氢存储量。原位傅立叶变换红外和11 H NMR技术证实了吸氢后AcP的氢化和脱氢形式。H 2的分压和温度可以容易地调节可逆的氢化/脱氢。仅当AcP和Pd NP相邻存在时,才能完成化学氢存储。每个循环中Pd的负载量增加至0.33 mmol H 2 ·g –1时,化学氢存储量得到了增强。通过控制150°C的氢化和脱氢温度,化学氢的存储量最多可维持10个循环。本文报道的材料是可在恒定温度和大气压下运行的非低温化学氢存储装置之一。











































 京公网安备 11010802027423号
京公网安备 11010802027423号