当前位置:
X-MOL 学术
›
ACS Appl. Bio Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Remodeling of Three-Dimensional Collagen I Matrices by Human Bone Marrow Stromal Cells during Osteogenic Differentiation In Vitro
ACS Applied Bio Materials ( IF 4.6 ) Pub Date : 2020-09-08 , DOI: 10.1021/acsabm.0c00856 Sarah Vogel 1 , Franziska Ullm 2 , Claudia Damaris Müller 1 , Tilo Pompe 2 , Ute Hempel 1
ACS Applied Bio Materials ( IF 4.6 ) Pub Date : 2020-09-08 , DOI: 10.1021/acsabm.0c00856 Sarah Vogel 1 , Franziska Ullm 2 , Claudia Damaris Müller 1 , Tilo Pompe 2 , Ute Hempel 1
Affiliation
|
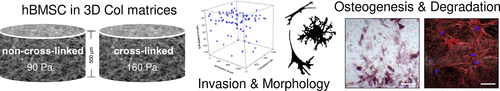
Cell fate is triggered by the characteristics of the surrounding extracellular matrix (ECM) including its composition and topological and mechanical properties. Human bone marrow stromal cells (hBMSC) are known to reside in a niche environment where they are maintained in a quiescent, multipotent state, also controlled by the ECM characteristics. In this in vitro study, three-dimensional (3D) fibrillary collagen I (Col)-based matrices with defined topological and mechanical characteristics were used (pore size of 3–4 μm, fibril diameter of ∼0.7 μm, ∼90 Pa (non-cross-linked), and ∼160 Pa (cross-linked)), mimicking conditions of the environment in the bone marrow. The performance of non-cross-linked and cross-linked scaffolds during osteogenic differentiation of hBMSC in terms of matrix stiffness and proteolytic degradability was investigated. Cell adhesion, morphology, and invasion as well as matrix remodeling were investigated on cross-linked and non-cross-linked Col matrices over 22 days. About 25% of the cells invaded the matrices and showed a spread morphology independent of cross-linking. Cellular proteolytic matrix degradation in terms of a decreased matrix layer thickness was only found for non-cross-linked matrices at constant pore size and fibril diameter. Osteogenic differentiation of hBMSC was examined by alkaline phosphatase staining and enzyme activity (early marker) and calcium phosphate deposition (late marker) and was similarly supported in both scaffolds. Furthermore, both matrices were strongly stiffened by about 10-fold because of high mineralization under osteogenic conditions. In summary, these results emphasize that fibrillary 3D Col matrices are a suitable model to study primary hBMSC behavior in terms of ECM remodeling during osteogenesis at defined in vitro conditions.
中文翻译:

体外成骨分化过程中人骨髓基质细胞对三维胶原蛋白 I 基质的重塑
细胞命运是由周围细胞外基质 (ECM) 的特征触发的,包括其组成以及拓扑和机械特性。已知人骨髓基质细胞 (hBMSC) 存在于一个生态位环境中,在那里它们维持在静止的多能状态,也受 ECM 特性的控制。在这个体外研究中,使用具有定义的拓扑和机械特性的三维 (3D) 纤维胶原蛋白 I (Col) 基质(孔径为 3-4 μm,原纤维直径为 ∼0.7 μm,∼90 Pa(非交联)和~160 Pa(交联)),模拟骨髓中的环境条件。研究了非交联和交联支架在 hBMSC 成骨分化过程中在基质刚度和蛋白水解降解性方面的性能。在 22 天内对交联和非交联的 Col 基质进行了细胞粘附、形态和侵袭以及基质重塑的研究。大约 25% 的细胞侵入基质并显示出与交联无关的扩散形态。仅在恒定孔径和原纤维直径的非交联基质中发现细胞蛋白水解基质降解方面的基质层厚度降低。通过碱性磷酸酶染色和酶活性(早期标记)和磷酸钙沉积(晚期标记)检查 hBMSC 的成骨分化,并且在两种支架中同样得到支持。此外,由于成骨条件下的高矿化作用,两种基质都强烈硬化了约 10 倍。总之,这些结果强调了纤维状 3D Col 矩阵是研究原发性 hBMSC 在成骨过程中 ECM 重塑行为的合适模型。通过碱性磷酸酶染色和酶活性(早期标记)和磷酸钙沉积(晚期标记)检查 hBMSC 的成骨分化,并且在两种支架中同样得到支持。此外,由于成骨条件下的高矿化作用,两种基质都强烈硬化了约 10 倍。总之,这些结果强调了纤维状 3D Col 矩阵是研究原发性 hBMSC 在成骨过程中 ECM 重塑行为的合适模型。通过碱性磷酸酶染色和酶活性(早期标记)和磷酸钙沉积(晚期标记)检查 hBMSC 的成骨分化,并且在两种支架中同样得到支持。此外,由于成骨条件下的高矿化作用,两种基质都强烈硬化了约 10 倍。总之,这些结果强调了纤维状 3D Col 矩阵是研究原发性 hBMSC 在成骨过程中 ECM 重塑行为的合适模型。体外条件。
更新日期:2020-10-21
中文翻译:

体外成骨分化过程中人骨髓基质细胞对三维胶原蛋白 I 基质的重塑
细胞命运是由周围细胞外基质 (ECM) 的特征触发的,包括其组成以及拓扑和机械特性。已知人骨髓基质细胞 (hBMSC) 存在于一个生态位环境中,在那里它们维持在静止的多能状态,也受 ECM 特性的控制。在这个体外研究中,使用具有定义的拓扑和机械特性的三维 (3D) 纤维胶原蛋白 I (Col) 基质(孔径为 3-4 μm,原纤维直径为 ∼0.7 μm,∼90 Pa(非交联)和~160 Pa(交联)),模拟骨髓中的环境条件。研究了非交联和交联支架在 hBMSC 成骨分化过程中在基质刚度和蛋白水解降解性方面的性能。在 22 天内对交联和非交联的 Col 基质进行了细胞粘附、形态和侵袭以及基质重塑的研究。大约 25% 的细胞侵入基质并显示出与交联无关的扩散形态。仅在恒定孔径和原纤维直径的非交联基质中发现细胞蛋白水解基质降解方面的基质层厚度降低。通过碱性磷酸酶染色和酶活性(早期标记)和磷酸钙沉积(晚期标记)检查 hBMSC 的成骨分化,并且在两种支架中同样得到支持。此外,由于成骨条件下的高矿化作用,两种基质都强烈硬化了约 10 倍。总之,这些结果强调了纤维状 3D Col 矩阵是研究原发性 hBMSC 在成骨过程中 ECM 重塑行为的合适模型。通过碱性磷酸酶染色和酶活性(早期标记)和磷酸钙沉积(晚期标记)检查 hBMSC 的成骨分化,并且在两种支架中同样得到支持。此外,由于成骨条件下的高矿化作用,两种基质都强烈硬化了约 10 倍。总之,这些结果强调了纤维状 3D Col 矩阵是研究原发性 hBMSC 在成骨过程中 ECM 重塑行为的合适模型。通过碱性磷酸酶染色和酶活性(早期标记)和磷酸钙沉积(晚期标记)检查 hBMSC 的成骨分化,并且在两种支架中同样得到支持。此外,由于成骨条件下的高矿化作用,两种基质都强烈硬化了约 10 倍。总之,这些结果强调了纤维状 3D Col 矩阵是研究原发性 hBMSC 在成骨过程中 ECM 重塑行为的合适模型。体外条件。










































 京公网安备 11010802027423号
京公网安备 11010802027423号