当前位置:
X-MOL 学术
›
Batteries Supercaps
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chloride Ion as Redox Mediator in Reducing Charge Overpotential of Aprotic Lithium‐Oxygen Batteries
Batteries & Supercaps ( IF 5.1 ) Pub Date : 2020-09-08 , DOI: 10.1002/batt.202000198 Qi Zhang 1 , Yin Zhou 2 , Wenrui Dai 2 , Xinhang Cui 2 , Zhiyang Lyu 3 , Zheng Hu 4 , Wei Chen 5
Batteries & Supercaps ( IF 5.1 ) Pub Date : 2020-09-08 , DOI: 10.1002/batt.202000198 Qi Zhang 1 , Yin Zhou 2 , Wenrui Dai 2 , Xinhang Cui 2 , Zhiyang Lyu 3 , Zheng Hu 4 , Wei Chen 5
Affiliation
|
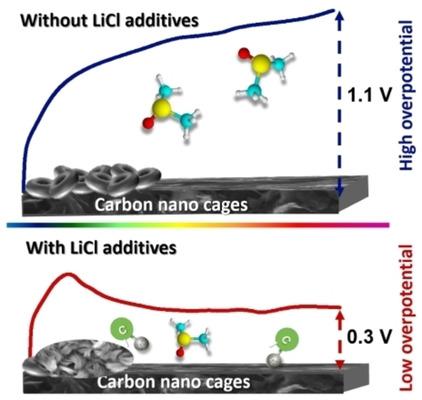
The aprotic lithium‐oxygen (Li−O2) battery with a high theoretical energy density has been considered as a promising candidate for next‐generation energy storage devices. However, the formation of insulating Li2O2 products is a major obstacle for realizing the high energy efficiency and long cycle life. Here, we report a new Cl−/Cl3− redox mediator to reduce the charge overpotential by a facile introduction of chloride ion (Cl−) additives into the organic electrolyte. The redox mediator can effectively promote the formation of the LiOH discharge product, and facilitate efficient LiOH decomposition. Therefore, the cell with the Cl− additives possesses a significantly low charge overpotential of 0.29 V, an extended cycle life (up to 71 cycles) at a rate of 500 mA g−1 with a fixed capacity of 500 mAh g−1, and an enhanced rate capability. This study offers an effective approach to modulate discharge products from Li2O2 to LiOH and provides new insights toward the role of redox mediators through the addition of Cl− in Li−O2 battery systems.
中文翻译:

氯离子作为氧化还原介体,减少非质子化锂氧电池的电荷超电势
具有高理论能量密度的非质子锂氧(Li-O 2)电池被认为是下一代储能设备的有希望的候选者。然而,绝缘Li 2 O 2产物的形成是实现高能量效率和长循环寿命的主要障碍。在这里,我们报告一个新氯- /氯3 -氧化还原介体,以减少电荷通过一个浅显的引入的氯离子(CL超电势- )添加剂加入到有机电解质。氧化还原介体可以有效地促进LiOH放电产物的形成,并促进有效的LiOH分解。因此,用氯细胞-添加剂具有0.29 V的极低电荷超电势,在500 mA g -1的速率下具有500 mAh g -1的固定容量,可延长循环寿命(最多71个循环),并具有增强的倍率能力。这项研究提供了一个有效的方法来调节放电产物李2 Ø 2至氢氧化锂,并提供对氧化还原调解员通过添加氯的作用,新的见解-李-O 2的电池系统。
更新日期:2020-09-08
中文翻译:

氯离子作为氧化还原介体,减少非质子化锂氧电池的电荷超电势
具有高理论能量密度的非质子锂氧(Li-O 2)电池被认为是下一代储能设备的有希望的候选者。然而,绝缘Li 2 O 2产物的形成是实现高能量效率和长循环寿命的主要障碍。在这里,我们报告一个新氯- /氯3 -氧化还原介体,以减少电荷通过一个浅显的引入的氯离子(CL超电势- )添加剂加入到有机电解质。氧化还原介体可以有效地促进LiOH放电产物的形成,并促进有效的LiOH分解。因此,用氯细胞-添加剂具有0.29 V的极低电荷超电势,在500 mA g -1的速率下具有500 mAh g -1的固定容量,可延长循环寿命(最多71个循环),并具有增强的倍率能力。这项研究提供了一个有效的方法来调节放电产物李2 Ø 2至氢氧化锂,并提供对氧化还原调解员通过添加氯的作用,新的见解-李-O 2的电池系统。











































 京公网安备 11010802027423号
京公网安备 11010802027423号