Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Fine‐tuning the activity and stability of an evolved enzyme active‐site through noncanonical amino‐acids
The FEBS Journal ( IF 5.5 ) Pub Date : 2020-09-08 , DOI: 10.1111/febs.15560 Henry C Wilkinson 1 , Paul A Dalby 1
The FEBS Journal ( IF 5.5 ) Pub Date : 2020-09-08 , DOI: 10.1111/febs.15560 Henry C Wilkinson 1 , Paul A Dalby 1
Affiliation
|
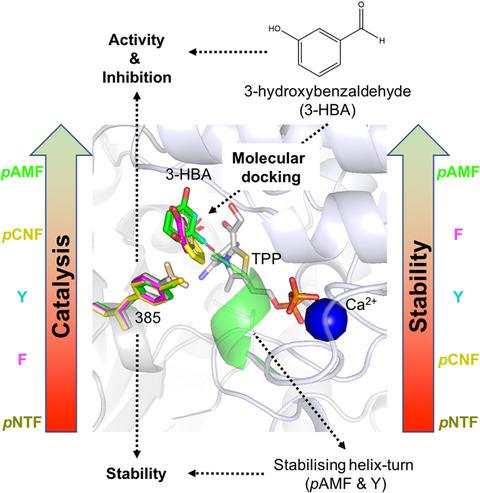
Site‐specific saturation mutagenesis within enzyme active sites can radically alter reaction specificity, though often with a trade‐off in stability. Extending saturation mutagenesis with a range of noncanonical amino acids (ncAA) potentially increases the ability to improve activity and stability simultaneously. Previously, an Escherichia coli transketolase variant (S385Y/D469T/R520Q) was evolved to accept aromatic aldehydes not converted by wild‐type. The aromatic residue Y385 was critical to the new acceptor substrate binding, and so was explored here beyond the natural aromatic residues, to probe side chain structure and electronics effects on enzyme function and stability. A series of five variants introduced decreasing aromatic ring electron density at position 385 in the order para‐aminophenylalanine (pAMF), tyrosine (Y), phenylalanine (F), para‐cyanophenylalanine (pCNF) and para‐nitrophenylalanine (pNTF), and simultaneously modified the hydrogen‐bonding potential of the aromatic substituent from accepting to donating. The fine‐tuning of residue 385 yielded variants with a 43‐fold increase in specific activity for 50 mm 3‐HBA and 100% increased kcat (pCNF), 290% improvement in Km (pNTF), 240% improvement in kcat/Km (pAMF) and decreased substrate inhibition relative to Y. Structural modelling suggested switching of the ring‐substituted functional group, from donating to accepting, stabilised a helix‐turn (D259‐H261) through an intersubunit H‐bond with G262, to give a 7.8 °C increase in the thermal transition mid‐point, Tm, and improved packing of pAMF. This is one of the first examples in which both catalytic activity and stability are simultaneously improved via site‐specific ncAA incorporation into an enzyme active site, and further demonstrates the benefits of expanding designer libraries to include ncAAs.
中文翻译:

通过非规范氨基酸微调进化后的酶活性位点的活性和稳定性
酶活性位点内的位点特异性饱和诱变可以从根本上改变反应特异性,尽管通常会在稳定性上进行权衡。使用一系列非规范氨基酸(ncAA)扩展饱和诱变可能会同时提高活性和稳定性。以前,一个大肠杆菌 大肠杆菌的转酮酶变体(S385Y / D469T / R520Q)放出接受不被野生型转化的芳香醛。芳香族残基Y385对新受体底物的结合至关重要,因此在这里我们探索了天然芳香族残基以外的地方,以探测侧链结构和电子对酶功能和稳定性的影响。一系列五个变体在385位顺序引入了降低芳环电子密度的顺序对氨基苯丙氨酸(p AMF),酪氨酸(Y),苯丙氨酸(F),对氰基苯丙氨酸(p CNF)和对硝基苯丙氨酸(p NTF),同时将芳族取代基的氢键势从接受到捐赠。残基385的微调产生了对50 m m 3-HBA的比活增加43倍且k cat(p CNF)增加100%,K m(p NTF)改善290%,240%改善的变体在k cat / K m(pAMF)和相对于Y降低的底物抑制作用。结构模型表明,环取代的官能团从供体到接受的转换,通过与G262的亚基间的H键使螺旋转角(D259-H261)稳定,得到7.8 °C的热转变中点T m升高,并且p AMF的堆积得到改善。这是第一个实例,其中通过将位点特异性ncAA掺入酶活性位点同时提高了催化活性和稳定性,并进一步证明了将设计器库扩展到包括ncAA的好处。
更新日期:2020-09-08
中文翻译:

通过非规范氨基酸微调进化后的酶活性位点的活性和稳定性
酶活性位点内的位点特异性饱和诱变可以从根本上改变反应特异性,尽管通常会在稳定性上进行权衡。使用一系列非规范氨基酸(ncAA)扩展饱和诱变可能会同时提高活性和稳定性。以前,一个大肠杆菌 大肠杆菌的转酮酶变体(S385Y / D469T / R520Q)放出接受不被野生型转化的芳香醛。芳香族残基Y385对新受体底物的结合至关重要,因此在这里我们探索了天然芳香族残基以外的地方,以探测侧链结构和电子对酶功能和稳定性的影响。一系列五个变体在385位顺序引入了降低芳环电子密度的顺序对氨基苯丙氨酸(p AMF),酪氨酸(Y),苯丙氨酸(F),对氰基苯丙氨酸(p CNF)和对硝基苯丙氨酸(p NTF),同时将芳族取代基的氢键势从接受到捐赠。残基385的微调产生了对50 m m 3-HBA的比活增加43倍且k cat(p CNF)增加100%,K m(p NTF)改善290%,240%改善的变体在k cat / K m(pAMF)和相对于Y降低的底物抑制作用。结构模型表明,环取代的官能团从供体到接受的转换,通过与G262的亚基间的H键使螺旋转角(D259-H261)稳定,得到7.8 °C的热转变中点T m升高,并且p AMF的堆积得到改善。这是第一个实例,其中通过将位点特异性ncAA掺入酶活性位点同时提高了催化活性和稳定性,并进一步证明了将设计器库扩展到包括ncAA的好处。











































 京公网安备 11010802027423号
京公网安备 11010802027423号