Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Involvement of subdomain II in the recognition of acetyl‐CoA revealed by the crystal structure of homocitrate synthase from Sulfolobus acidocaldarius
The FEBS Journal ( IF 5.5 ) Pub Date : 2020-09-08 , DOI: 10.1111/febs.15527 Tomohiro Suzuki 1 , Takeo Tomita 1, 2 , Kenta Hirayama 1 , Michio Suzuki 3 , Tomohisa Kuzuyama 1, 2 , Makoto Nishiyama 1, 2
The FEBS Journal ( IF 5.5 ) Pub Date : 2020-09-08 , DOI: 10.1111/febs.15527 Tomohiro Suzuki 1 , Takeo Tomita 1, 2 , Kenta Hirayama 1 , Michio Suzuki 3 , Tomohisa Kuzuyama 1, 2 , Makoto Nishiyama 1, 2
Affiliation
|
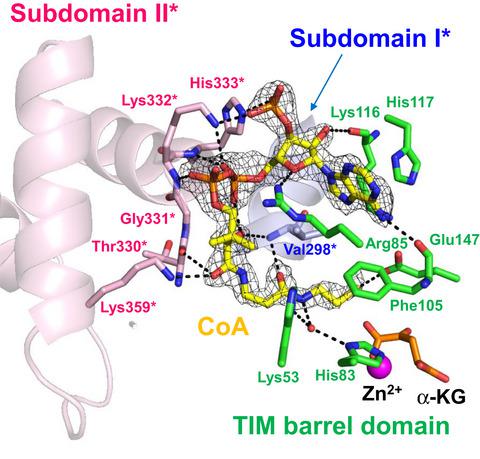
Homocitrate synthase (HCS) catalyzes the aldol condensation of α‐ketoglutarate and acetyl coenzyme A to form homocitrate, which is the first committed step of lysine biosynthesis through the α‐aminoadipate pathway in yeast, fungi, and some prokaryotes. We determined the crystal structure of a truncated form of HCS from a hyperthermophilic acidophilic archaeon, Sulfolobus acidocaldarius, which lacks the RAM (Regulation of amino acid metabolism) domain at the C terminus serving as the regulatory domain for the feedback inhibition by lysine, in complex with α‐ketoglutarate, Mg2+, and CoA. This structure coupled with mutational analysis revealed that a subdomain, subdomain II, connecting the N‐terminal catalytic domain and C‐terminal RAM domain is involved in the recognition of acetyl‐CoA. This is the first structural evidence of the function of subdomain II in the related enzyme family, which will lead to a better understanding of the catalytic mechanism of HCS.
中文翻译:

Sulfolobus acidocaldarius的纯柠檬酸合酶的晶体结构揭示了亚域II参与乙酰辅酶A的识别
纯柠檬酸合酶(HCS)催化α-酮戊二酸与乙酰辅酶A的醛醇缩合形成纯柠檬酸,这是通过酵母,真菌和某些原核生物中α-氨基己二酸途径进行赖氨酸生物合成的第一个重要步骤。我们确定HCS的截短形式的晶体结构从超嗜热嗜酸古细菌,嗜酸热硫化叶,它缺乏RAM(ř的egulation一个美浓酸米etabolism)结构域在C末端用作由赖氨酸反馈抑制的调控结构域,与α-酮戊二酸酯复合,Mg 2+和CoA。这种结构加上突变分析表明,连接N末端催化结构域和C末端RAM结构域的亚结构域II参与了乙酰辅酶A的识别。这是亚域II在相关酶家族中功能的第一个结构证据,这将导致人们对HCS的催化机理有更好的了解。
更新日期:2020-09-08
中文翻译:

Sulfolobus acidocaldarius的纯柠檬酸合酶的晶体结构揭示了亚域II参与乙酰辅酶A的识别
纯柠檬酸合酶(HCS)催化α-酮戊二酸与乙酰辅酶A的醛醇缩合形成纯柠檬酸,这是通过酵母,真菌和某些原核生物中α-氨基己二酸途径进行赖氨酸生物合成的第一个重要步骤。我们确定HCS的截短形式的晶体结构从超嗜热嗜酸古细菌,嗜酸热硫化叶,它缺乏RAM(ř的egulation一个美浓酸米etabolism)结构域在C末端用作由赖氨酸反馈抑制的调控结构域,与α-酮戊二酸酯复合,Mg 2+和CoA。这种结构加上突变分析表明,连接N末端催化结构域和C末端RAM结构域的亚结构域II参与了乙酰辅酶A的识别。这是亚域II在相关酶家族中功能的第一个结构证据,这将导致人们对HCS的催化机理有更好的了解。










































 京公网安备 11010802027423号
京公网安备 11010802027423号