当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photochemistry with Chlorine Trifluoride: Syntheses and Characterization of Difluorooxychloronium(V) Hexafluorido(non)metallates(V), [ClOF2][MF6] (M=V, Nb, Ta, Ru, Os, Ir, P, Sb)
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2020-09-08 , DOI: 10.1002/chem.202003629 Benjamin Scheibe 1 , Antti J Karttunen 2 , Florian Weigend 1 , Florian Kraus 1
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2020-09-08 , DOI: 10.1002/chem.202003629 Benjamin Scheibe 1 , Antti J Karttunen 2 , Florian Weigend 1 , Florian Kraus 1
Affiliation
|
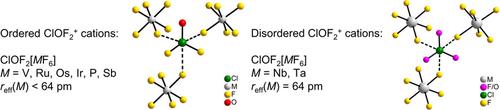
A photochemical route to salts consisting of difluorooxychloronium(V) cations, [ClOF2]+, and hexafluorido(non)metallate(V) anions, [MF6]− (M=V, Nb, Ta, Ru, Os, Ir, P, Sb) is presented. As starting materials, either metals, oxygen and ClF3 or oxides and ClF3 are used. The prepared compounds were characterized by single‐crystal X‐ray diffraction and Raman spectroscopy. The crystal structures of [ClOF2][MF6] (M=V, Ru, Os, Ir, P, Sb) are layer structures that are isotypic with the previously reported compound [ClOF2][AsF6], whereas for M=Nb and Ta, similar crystal structures with a different stacking variant of the layers are observed. Additionally, partial or full O/F disorder within the [ClOF2]+ cations of the Nb and Ta compounds occurs. In all compounds reported here, a trigonal pyramidal [ClOF2]+ cation with three additional Cl⋅⋅⋅F contacts to neighboring [MF6]− anions is observed, resulting in a pseudo‐octahedral coordination sphere around the Cl atom. The Cl−F and Cl−O bond lengths of the [ClOF2]+ cations seem to correlate with the effective ionic radii of the MV ions. Quantum‐chemical, solid‐state calculations well reproduce the experimental Raman spectra and show, as do quantum‐chemical gas phase calculations, that the secondary Cl⋅⋅⋅F interactions are ionic in nature. However, both solid‐state and gas‐phase quantum‐chemical calculations fail to reproduce the increases in the Cl−O bond lengths with increasing effective ionic radius of M in [MF6]− and the Cl−O Raman shifts also do not generally follow this trend.
中文翻译:

三氟化氯光化学:二氟氯鎓(V)六氟化(非)金属盐(V)、[ClOF2][MF6] (M=V、Nb、Ta、Ru、Os、Ir、P、Sb)的合成和表征
光化学途径生成由二氟氯鎓 (V) 阳离子 [ClOF 2 ] +和六氟(非)金属酸盐 (V) 阴离子 [ M F 6 ] − ( M =V, Nb, Ta, Ru, Os, Ir) 组成的盐,P,Sb)被提出。使用金属、氧和ClF 3或氧化物和ClF 3作为起始材料。通过单晶X射线衍射和拉曼光谱对所制备的化合物进行了表征。 [ClOF 2 ][ M F 6 ] ( M =V, Ru, Os, Ir, P, Sb) 的晶体结构是层状结构,与之前报道的化合物 [ClOF 2 ][AsF 6 ] 是同型的,而对于M = Nb 和 Ta,观察到具有不同层堆叠变体的相似晶体结构。另外,Nb和Ta化合物的[ClOF 2 ] +阳离子内发生部分或全部O/F紊乱。在此报告的所有化合物中,观察到三角锥体 [ClOF 2 ] +阳离子与邻近的 [ M F 6 ] -阴离子具有三个额外的 Cl⋅⋅⋅F 接触,从而在 Cl 原子周围形成伪八面体配位球。 [ClOF 2 ] +阳离子的 Cl−F 和 Cl−O 键长似乎与M V离子的有效离子半径相关。量子化学、固态计算很好地再现了实验拉曼光谱,并且与量子化学气相计算一样,表明次级 Cl⋅⋅⋅F 相互作用本质上是离子性的。 然而,固态和气相量子化学计算都无法重现随着 [ M F 6 ] -中M有效离子半径的增加,Cl−O 键长的增加,并且 Cl−O 拉曼位移也没有重现。一般都会遵循这个趋势。
更新日期:2020-09-08
中文翻译:

三氟化氯光化学:二氟氯鎓(V)六氟化(非)金属盐(V)、[ClOF2][MF6] (M=V、Nb、Ta、Ru、Os、Ir、P、Sb)的合成和表征
光化学途径生成由二氟氯鎓 (V) 阳离子 [ClOF 2 ] +和六氟(非)金属酸盐 (V) 阴离子 [ M F 6 ] − ( M =V, Nb, Ta, Ru, Os, Ir) 组成的盐,P,Sb)被提出。使用金属、氧和ClF 3或氧化物和ClF 3作为起始材料。通过单晶X射线衍射和拉曼光谱对所制备的化合物进行了表征。 [ClOF 2 ][ M F 6 ] ( M =V, Ru, Os, Ir, P, Sb) 的晶体结构是层状结构,与之前报道的化合物 [ClOF 2 ][AsF 6 ] 是同型的,而对于M = Nb 和 Ta,观察到具有不同层堆叠变体的相似晶体结构。另外,Nb和Ta化合物的[ClOF 2 ] +阳离子内发生部分或全部O/F紊乱。在此报告的所有化合物中,观察到三角锥体 [ClOF 2 ] +阳离子与邻近的 [ M F 6 ] -阴离子具有三个额外的 Cl⋅⋅⋅F 接触,从而在 Cl 原子周围形成伪八面体配位球。 [ClOF 2 ] +阳离子的 Cl−F 和 Cl−O 键长似乎与M V离子的有效离子半径相关。量子化学、固态计算很好地再现了实验拉曼光谱,并且与量子化学气相计算一样,表明次级 Cl⋅⋅⋅F 相互作用本质上是离子性的。 然而,固态和气相量子化学计算都无法重现随着 [ M F 6 ] -中M有效离子半径的增加,Cl−O 键长的增加,并且 Cl−O 拉曼位移也没有重现。一般都会遵循这个趋势。











































 京公网安备 11010802027423号
京公网安备 11010802027423号