Journal of CO2 Utilization ( IF 7.2 ) Pub Date : 2020-09-08 , DOI: 10.1016/j.jcou.2020.101264 Alicia Bayón , Víctor A. de la Peña O’Shea , David P. Serrano , Juan M. Coronado
|
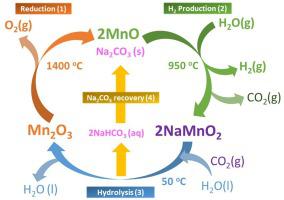
Thermochemical cycles for H2O and CO2 splitting using CeO2 have demonstrated high efficiency (> 5%) and fast kinetics. Alternative multistage cycles based on stoichiometric oxides, such as Mn-Na, can potentially provide much larger efficiencies under less stringent operation conditions. However, these cycles consist of three stages operating at very different temperatures (from 50 °C to 1500 °C) and, accordingly, the overall energy yield of the cycle critically depends on efficient heat recovery. Despite of this, recently the Mn3O4–Na2CO3 cycle has shown a remarkable H2 production and high stability. To further enhance hydrogen generation rate, in this work we explore the feasibility of the novel cycle based on MnO, which can potentially increase the yield per cycle. Several samples of MnO with different physicochemical characteristics were investigated to establish the influence on the oxide physicochemical properties on hydrogen generation. A maximum conversion of 47 %, with a productivity of 20.1 μmol H2 min−1 g−1 were obtained using the MnO sample with relatively large surface area. As expected, this productivity is notably better than that obtained with Mn3O4 under identical conditions. Furthermore, the results obtained here reveals that low temperature hydrolysis under CO2 atmosphere can improve the Na extraction by more than 10 %. This enhances the cyclability of the process and it may allow coupling hydrogen production with CO2 capture. Furthermore, the thermal reduction of the solid product recovered after the carbonation stage yields nearly pure MnO, confirming experimental the feasibility of completing this alternative cycle.
中文翻译:

探索用于水分解的替代MnO-Na 2 CO 3热化学循环
用于h热化学循环2 O和CO 2使用的CeO分裂2已经证明高效率(> 5%)和快的动力学。在较不严格的操作条件下,基于化学计量的氧化物(例如Mn-Na)的替代性多级循环可能会提供更高的效率。但是,这些循环由三个阶段组成,它们在非常不同的温度(从50°C到1500°C)下运行,因此,循环的总能量产出关键取决于有效的热回收。尽管如此,最近Mn 3 O 4 -Na 2 CO 3循环显示出了显着的H 2生产稳定度高。为了进一步提高氢气的产生速率,在这项工作中,我们探索了基于MnO的新型循环的可行性,这可能会提高每个循环的产率。研究了几种具有不同理化特性的MnO样品,以建立对氧化物理化特性对氢生成的影响。使用具有相对大表面积的MnO样品,获得了47%的最大转化率和20.1μmolH 2 min -1 g -1的生产率。如预期的那样,该生产率明显优于使用Mn 3 O 4所获得的生产率。在相同条件下。此外,这里获得的结果表明,在CO 2气氛下的低温水解可以将Na提取提高10%以上。这增强了过程的可循环性,并且可以使制氢与CO 2捕集耦合。此外,碳酸化阶段后回收的固体产物的热还原产生了近乎纯净的MnO,从而证实了完成该替代循环的实验可行性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号