Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2020-09-08 , DOI: 10.1016/j.bmcl.2020.127537 Duong Tien Anh 1 , Pham-The Hai 1 , Do Thi Mai Dung 1 , Phan Thi Phuong Dung 1 , Le-Thi-Thu Huong 1 , Eun Jae Park 2 , Hye Won Jun 2 , Jong Soon Kang 3 , Joo-Hee Kwon 3 , Truong Thanh Tung 4 , Sang-Bae Han 2 , Nguyen-Hai Nam 1
|
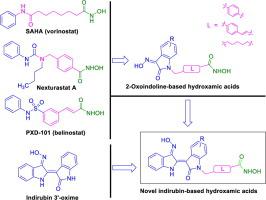
Several novel indirubin-based N-hydroxybenzamides, N-hydropropenamides and N-hydroxyheptanamides (4a-h, 7a-h, 10a-h) were designed using a fragment-based approach with structural features extracted from several previously reported HDAC inhibitors, such as SAHA (vorinostat), MGCD0103 (mocetinostat), nexturastat A and PXD-101 (belinostat). The biological results reveal that our compounds showed excellent cytotoxicity toward three common human cancer cell lines (SW620, PC-3 and NCI-H23) with IC50 values ranging from 0.09 to 0.007 µM. The cytotoxicity of the compounds was equipotent or even up to 10-times more potent than adriamycin and up to 205-times more potent than SAHA. Among the series of N-hydroxypropenamides, compounds 10a-d were the most potent HDAC inhibitors as well as cytotoxicity toward the cell lines tested. In addition, the strong inhibitory activites toward HDAC of our compounds were observed with IC50 values of below-micromolar range. Especially, compound 4a inhibited HDAC6 with an IC50 value of 29-fold lower than that against HDAC2 isoform. Representative compounds 4a and 7a were found to significantly arrest SW620 cells at G0/G1 phase. Compounds 7a and 10a were found to strongly induce apoptosis in SW620 cells. Docking studies revealed some important features affecting the selectivity against HDAC6 isoform. The results clearly demonstrate the potential of the indirubin-hydroxamic acid hybrids and these compounds should be very promising for further development.
中文翻译:

设计,合成和评估新型的基于靛玉红的N-羟基苯甲酰胺,N-羟基丙烯酰胺和N-羟基庚酰胺作为组蛋白脱乙酰基酶抑制剂和抗肿瘤剂。
使用基于片段的方法设计了几种新颖的基于靛玉红的N-羟基苯甲酰胺,N-氢丙烯酰胺和N-羟基庚酰胺(4a-h,7a-h,10a-h),其结构特征是从几种先前报道的HDAC抑制剂中提取的,例如SAHA(伏立诺他),MGCD0103(mocetinostat),nexturastat A和PXD-101(belinostat)。生物学结果表明,我们的化合物对三种常见的具有IC 50的人类癌细胞系(SW620,PC-3和NCI-H23)显示出优异的细胞毒性值范围从0.09到0.007 µM。化合物的细胞毒性是同等效力的,甚至比阿霉素的效力高达10倍,比SAHA的效力高达205倍。在一系列N-羟基丙烯酰胺中,化合物10a - d是最有效的HDAC抑制剂,并且对测试的细胞系具有细胞毒性。此外,我们观察到我们化合物对HDAC的强抑制活性,其IC 50值低于微摩尔范围。特别是,化合物4a抑制HDAC6的IC 50值比对抗HDAC2同工型的IC 50值低29倍。代表性化合物4a和7a被发现可将SW620细胞阻滞在G0 / G1期。发现化合物7a和10a强烈诱导SW620细胞中的细胞凋亡。对接研究揭示了一些重要特征,这些特征会影响针对HDAC6同工型的选择性。结果清楚地表明了靛玉红-异羟肟酸杂化物的潜力,并且这些化合物对于进一步开发应该是很有前途的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号