当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An organocatalytic domino Michael addition strategy: construction of bispiro[oxindole-thiazolidinone-hexahydroxanthone]s with five contiguous stereocenters.
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020-09-07 , DOI: 10.1039/d0ob01613f Yong-Xing Song 1 , Ye Lin 1 , Li Yan 2 , Da-Ming Du 1
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020-09-07 , DOI: 10.1039/d0ob01613f Yong-Xing Song 1 , Ye Lin 1 , Li Yan 2 , Da-Ming Du 1
Affiliation
|
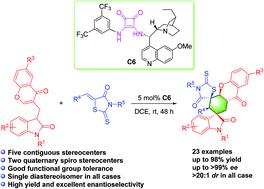
We herein report a highly efficient strategy for the stereoselective synthesis of structurally complex bispiro[oxindole-thiazolidinone-hexahydroxanthone]s. This squaramide-catalyzed domino Michael addition afforded these products in good to excellent yields with excellent stereoselectivities bearing five contiguous stereocenters with two spiroquaternary stereocenters. Meanwhile, both gram-scale synthesis and further transformation experiments have been also demonstrated.
中文翻译:

有机催化多米诺迈克尔加成策略:构建具有五个连续立体中心的双螺环[oxindole-thiazolidinone-hexahydroxanthone]。
我们在此报告了一种用于立体选择性合成结构复杂的双螺[羟吲哚-噻唑烷酮-六羟基蒽酮]s 的高效策略。这种方方酰胺催化的多米诺迈克尔加成使这些产品具有良好的产率和优异的立体选择性,具有五个连续的立体中心和两个螺季铵盐立体中心。同时,还展示了克级合成和进一步的转化实验。
更新日期:2020-09-30
中文翻译:

有机催化多米诺迈克尔加成策略:构建具有五个连续立体中心的双螺环[oxindole-thiazolidinone-hexahydroxanthone]。
我们在此报告了一种用于立体选择性合成结构复杂的双螺[羟吲哚-噻唑烷酮-六羟基蒽酮]s 的高效策略。这种方方酰胺催化的多米诺迈克尔加成使这些产品具有良好的产率和优异的立体选择性,具有五个连续的立体中心和两个螺季铵盐立体中心。同时,还展示了克级合成和进一步的转化实验。











































 京公网安备 11010802027423号
京公网安备 11010802027423号