Medicinal Chemistry ( IF 1.9 ) Pub Date : 2020-08-31 , DOI: 10.2174/1573406415666190612150447 Hayat Ullah 1 , Fazal Rahim 1 , Muhammad Taha 2 , Raffaqat Hussain 1 , Abdul Wadood 3 , Mohsan Nawaz 1 , Zainul Wahab 4 , Kanwal 5 , Khalid M. Khan 5
|
Background: In the recent past, we have synthesized and reported different derivatives of oxadiazoles as potential α-glucosidase inhibitors, keeping in mind, the pharmacological aspects of oxadiazole moiety and in continuation of our ongoing research on the chemistry and bioactivity of new heterocyclic compounds.
Methods: 1,3,4-Oxadiazole derivatives (1-14) have been synthesized and characterized by different spectroscopic techniques such as 1H-, 13C-NMR and HREI-MS.
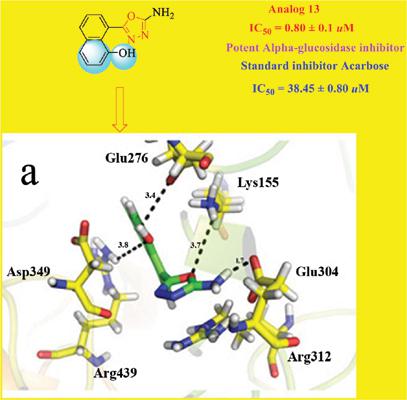
Results: The synthetic derivatives were screened for α-glucosidase inhibitory potential. All compounds exhibited good inhibitory activity with IC50 values ranging between 0.80 ± 0.1 to 45.1 ± 1.7 μM in comparison with the standard acarbose having IC50 value 38.45 ± 0.80 μM.
Conclusion: Thirteen compounds 1-6 and 8-14 showed potential inhibitory activity as compared to the standard acarbose having IC50 value 38.45 ± 0.80 μM, however, only one compound 7 (IC50 = 45.1 ± 1.7 μM) was found to be less active. Compound 14 (IC50 = 0.80 ± 0.1 μM) showed promising inhibitory activity among all synthetic derivatives. Molecular docking studies were also conducted for the active compounds to understand the ligand-enzyme binding interactions.
中文翻译:

2-氨基-1,3,4-恶二唑衍生物的合成,体外α-葡萄糖苷酶抑制潜能和分子对接研究
背景:在最近的研究中,我们牢记了恶二唑基团的药理作用,并在继续进行有关新型杂环化合物的化学和生物活性的研究的过程中,合成并报道了恶二唑类化合物作为潜在的α-葡萄糖苷酶抑制剂的不同衍生物。
方法:合成了1,3,4-恶二唑衍生物(1-14),并通过1H-,13C-NMR和HREI-MS等不同的光谱技术对其进行了表征。
结果:筛选了合成衍生物的α-葡萄糖苷酶抑制潜力。与具有IC50值38.45±0.80μM的标准阿卡波糖相比,所有化合物均表现出良好的抑制活性,IC50值在0.80±0.1至45.1±1.7μM之间。
结论:与IC50值为38.45±0.80μM的标准阿卡波糖相比,十三种化合物1-6和8-14具有潜在的抑制活性,但是,仅发现一种化合物7(IC50 = 45.1±1.7μM)活性较低。在所有合成衍生物中,化合物14(IC50 = 0.80±0.1μM)显示出有希望的抑制活性。还对活性化合物进行了分子对接研究,以了解配体-酶结合相互作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号