Medicinal Chemistry ( IF 1.9 ) Pub Date : 2020-08-31 , DOI: 10.2174/1573406416666191227113716 Michelyne Haroun 1
|
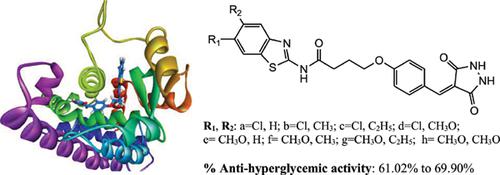
Background: The discovery of novel ligand binding domain (LBD) of peroxisome proliferator- activated receptor γ (PPARγ) has recently attracted attention to few research groups in order to develop more potent and safer antidiabetic agents.
Objective: This study is focused on docking-based design and synthesis of novel compounds combining benzothiazole and pyrazolidinedione scaffold as potential antidiabetic agents.
Methods: Several benzothiazole-pyrazolidinedione hybrids were synthesized and tested for their in vivo anti-hyperglycemic activity. Interactions profile of title compounds against PPARγ was examined through molecular modelling approach.
Results: All tested compounds exhibited anti-hyperglycemic activity similar or superior to the reference drug Rosiglitazone. Introducing chlorine atom and alkyl group at position-6 and -5 respectively on benzothiazole core resulted in enhancing the anti-hyperglycemic effect. Docking study revealed that such groups demonstrated favorable hydrophobic interactions with novel LBD Ω- pocket of PPARγ protein.
Conclusion: Among the tested compounds, N-(6-chloro-5-methylbenzo[d]thiazol-2-yl-4-(4((3,5- dioxopyrazolidin-4-ylidene)methyl)phenoxy)butanamide 5b was found to be the most potent compound and provided valuable insights to further develop novel hybrids as anti-hyperglycemic agents.
中文翻译:

在计算机上设计,合成和评估新型的基于苯并噻唑的吡唑烷二酮类强效降血糖药
背景:过氧化物酶体增殖物激活的受体γ(PPARγ)的新型配体结合域(LBD)的发现最近吸引了少数研究小组的注意,以开发更有效和更安全的抗糖尿病药。
目的:本研究专注于基于对接的设计和合成结合苯并噻唑和吡唑烷二酮骨架作为潜在抗糖尿病药的新型化合物。
方法:合成了几种苯并噻唑-吡唑烷二酮杂化物,并测试了它们的体内抗高血糖活性。通过分子建模方法研究了标题化合物与PPARγ的相互作用情况。
结果:所有测试的化合物均显示出与参考药物罗格列酮相似或更高的抗高血糖活性。在苯并噻唑核心上分别在-6位和-5位引入氯原子和烷基导致增强的降血糖作用。对接研究表明,这些基团与新型PPARγ蛋白的LBDΩ-口袋具有良好的疏水性相互作用。
结论:在被测化合物中,发现了N-(6-氯-5-甲基苯并[d]噻唑-2-基-4-(4((3,5-二氧杂吡唑并丁-4-亚基)甲基)苯氧基)丁酰胺5b是最有效的化合物,并提供了宝贵的见识,可进一步开发出新型的抗高血压药。











































 京公网安备 11010802027423号
京公网安备 11010802027423号