当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Di-tert-butylphosphate Derived Thermolabile Calcium Organophosphates: Precursors for Ca(H2PO4)2, Ca(HPO4), α-/β-Ca(PO3)2, and Nanocrystalline Ca10(PO4)6(OH)2.
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2020-09-07 , DOI: 10.1021/acs.inorgchem.0c01591 Sonam Verma 1 , Ramaswamy Murugavel 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2020-09-07 , DOI: 10.1021/acs.inorgchem.0c01591 Sonam Verma 1 , Ramaswamy Murugavel 1
Affiliation
|
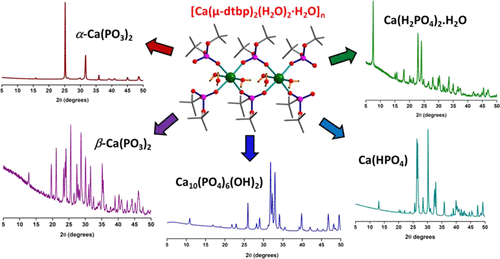
Thermally and hydrolytically unstable di-tert-butyl phosphate (dtbp-H) has been used as synthon to prepare discrete and polymeric calcium phosphates that are convenient single-source precursors for a range of calcium phosphate ceramic biomaterials. The reactivity of dtbp-H toward two different calcium sources has been found to vary significantly, e.g., the reaction of Ca(OMe)2 with dtbp-H in a 1:6 molar ratio in petroleum ether forms a mononuclear calcium hexa-phosphate complex [Ca(dtbp)2(dtbp-H)4] (1), whereas the change of calcium source to CaH2, in a 1:2 molar ratio under otherwise similar reaction conditions, yields the calcium phosphate polymer, [Ca(μ-dtbp)2(H2O)2·H2O]n(2). Compounds 1 and 2 have been extensively characterized by various spectroscopic and analytical techniques. The solid-state structures of both 1 and 2 have been determined by single-crystal X-ray diffraction studies. In discrete molecule 1, the central calcium ion is surrounded by two anionic dtbp and four neutral dtbp-H ligands in an octahedral coordination environment. Compound 2 is a one-dimensional polymer in which adjacent calcium ions are connected through double dtbp bridges. Solid-state thermolysis of bulk 1 in air leads to the exclusive formation of calcium metaphosphate β-Ca(PO3)2 in the entire temperature range of 400–800 °C. Thermal decomposition of polymer 2, however, can be fine-tuned to produce either α-Ca(PO3)2 or β-Ca(PO3)2 depending on the thermolysis conditions employed. Although the sample sintered at 600 °C produces exclusively α-form of Ca(PO3)2, the sample annealed at 800 °C or above produces β-form. Both α- and β-forms can also be successively formed one after other by a slow heating of a freshly prepared 2 on the powder diffractometer sample holder. Additional forms of ceramic phosphates have been prepared by solvothermal conditions because of the highly labile nature of the tert-butoxy groups of dtbp in 1 and 2. Solution decomposition of either 1 or 2 in boiling toluene at 140 °C in a sealed tube produces calcium dihydrogen phosphate [Ca(H2PO4)2·H2O] as the only product in the form of single crystals. Solution thermolysis of 2 in protic solvents such as water and methanol can be biased to produce other calcium phosphate biomaterials such as hydroxyapatite [Ca10(PO4)6(OH)2]and calcium monohydrogen phosphate [Ca(HPO4)] in the presence of additional calcium precursors such as CaO and Ca(OMe)2, respectively.
中文翻译:

磷酸二叔丁酯衍生的热不稳定有机磷酸钙:Ca(H2PO4)2,Ca(HPO4),α-/β-Ca(PO3)2和纳米晶Ca10(PO4)6(OH)2的前体。
热和水解不稳定的磷酸二叔丁酯(dtbp-H)已被用作合成子,以制备离散的和聚合的磷酸钙,它们是一系列磷酸钙陶瓷生物材料的方便的单源前体。已发现dtbp-H对两种不同钙源的反应性显着变化,例如,Ca(OMe)2与dtbp-H在石油醚中的摩尔比为1:6的反应形成了单核六磷酸钙复合物[Ca(dtbp)2(dtbp-H)4 ](1),而在其他相似的反应条件下,以1:2的摩尔比将钙源改变为CaH 2,则会得到磷酸钙聚合物[Ca(μ -dtbp)2(H 2 O)2 · H 2 O] n(2)。化合物1和2已通过各种光谱和分析技术进行了广泛表征。1和2的固态结构已通过单晶X射线衍射研究确定。在离散分子1中,中央钙离子在八面体配位环境中被两个阴离子dtbp和四个中性dtbp-H配体包围。化合物2是一维聚合物,其中相邻的钙离子通过双dtbp桥连接。固体的固态热解在空气中1会导致在整个400-800°C的温度范围内独家形成偏磷酸钙β-Ca(PO 3)2。然而,取决于所采用的热解条件,可以对聚合物2的热分解进行微调以产生α-Ca(PO 3)2或β-Ca(PO 3)2。尽管在600℃下烧结的样品仅产生α形式的Ca(PO 3)2,但是在800℃或更高温度下退火的样品则产生β形式。通过缓慢加热新鲜制备的2, α和β形式也可以依次形成。在粉末衍射仪样品架上。由于dtbp的叔丁氧基在1和2中的高度不稳定的性质,已经通过溶剂热条件制备了其他形式的陶瓷磷酸盐。在密封管中在140°C的沸腾甲苯中将1或2进行溶液分解,生成磷酸二氢钙[Ca(H 2 PO 4)2 · H 2 O]作为单晶形式的唯一产物。在质子性溶剂(例如水和甲醇)中溶液2的溶液热解可产生其他磷酸钙生物材料,例如羟基磷灰石[Ca10(PO 4)6(OH)2 ]和磷酸一氢钙[Ca(HPO 4)],分别存在其他钙前体,例如CaO和Ca(OMe)2。
更新日期:2020-09-21
中文翻译:

磷酸二叔丁酯衍生的热不稳定有机磷酸钙:Ca(H2PO4)2,Ca(HPO4),α-/β-Ca(PO3)2和纳米晶Ca10(PO4)6(OH)2的前体。
热和水解不稳定的磷酸二叔丁酯(dtbp-H)已被用作合成子,以制备离散的和聚合的磷酸钙,它们是一系列磷酸钙陶瓷生物材料的方便的单源前体。已发现dtbp-H对两种不同钙源的反应性显着变化,例如,Ca(OMe)2与dtbp-H在石油醚中的摩尔比为1:6的反应形成了单核六磷酸钙复合物[Ca(dtbp)2(dtbp-H)4 ](1),而在其他相似的反应条件下,以1:2的摩尔比将钙源改变为CaH 2,则会得到磷酸钙聚合物[Ca(μ -dtbp)2(H 2 O)2 · H 2 O] n(2)。化合物1和2已通过各种光谱和分析技术进行了广泛表征。1和2的固态结构已通过单晶X射线衍射研究确定。在离散分子1中,中央钙离子在八面体配位环境中被两个阴离子dtbp和四个中性dtbp-H配体包围。化合物2是一维聚合物,其中相邻的钙离子通过双dtbp桥连接。固体的固态热解在空气中1会导致在整个400-800°C的温度范围内独家形成偏磷酸钙β-Ca(PO 3)2。然而,取决于所采用的热解条件,可以对聚合物2的热分解进行微调以产生α-Ca(PO 3)2或β-Ca(PO 3)2。尽管在600℃下烧结的样品仅产生α形式的Ca(PO 3)2,但是在800℃或更高温度下退火的样品则产生β形式。通过缓慢加热新鲜制备的2, α和β形式也可以依次形成。在粉末衍射仪样品架上。由于dtbp的叔丁氧基在1和2中的高度不稳定的性质,已经通过溶剂热条件制备了其他形式的陶瓷磷酸盐。在密封管中在140°C的沸腾甲苯中将1或2进行溶液分解,生成磷酸二氢钙[Ca(H 2 PO 4)2 · H 2 O]作为单晶形式的唯一产物。在质子性溶剂(例如水和甲醇)中溶液2的溶液热解可产生其他磷酸钙生物材料,例如羟基磷灰石[Ca10(PO 4)6(OH)2 ]和磷酸一氢钙[Ca(HPO 4)],分别存在其他钙前体,例如CaO和Ca(OMe)2。











































 京公网安备 11010802027423号
京公网安备 11010802027423号