当前位置:
X-MOL 学术
›
ChemPhysChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rivalry between regium and hydrogen bonds established within diatomic coinage molecules and Lewis acids/bases.
ChemPhysChem ( IF 2.3 ) Pub Date : 2020-09-06 , DOI: 10.1002/cphc.202000704 Goar Sánchez-Sanz 1 , Cristina Trujillo 2 , Ibon Alkorta 3 , José Elguero 3
ChemPhysChem ( IF 2.3 ) Pub Date : 2020-09-06 , DOI: 10.1002/cphc.202000704 Goar Sánchez-Sanz 1 , Cristina Trujillo 2 , Ibon Alkorta 3 , José Elguero 3
Affiliation
|
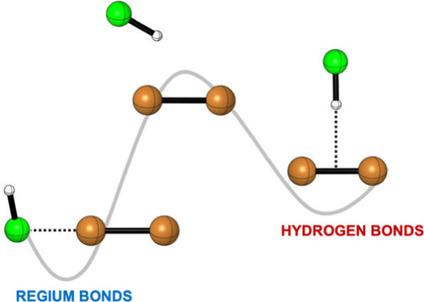
A theoretical study of the complexes formed by Ag2 and Cu2 with different molecules, XH (FH, ClH, OH2, SH2, HCN, HNC, HCCH, NH3 and PH3) that can act as hydrogen‐bond donors (Lewis acids) or regium‐bond acceptors (Lewis bases) was carried out at the CCSD(T)/CBS computational level. The heteronuclear diatomic coinage molecules (AuAg, AuCu, and AgCu) have also been considered. With the exception of some of the hydrogen‐bonded complexes with FH, the regium‐bonded binary complexes are more stable. The AuAg and AuCu molecules show large dipole moments that weaken the regium bond (RB) with Au and favour those through the Ag and Cu atoms, respectively.
中文翻译:

在双原子造币分子和路易斯酸/碱中建立的区域和氢键之间的竞争。
对由Ag 2和Cu 2与不同分子XH(FH,ClH,OH 2,SH 2,HCN,HNC,HCCH,NH 3和PH 3)形成的可作为氢键供体的配合物的理论研究(路易斯酸)或区域键受体(路易斯碱)是在CCSD(T)/ CBS计算级别上进行的。还考虑了异核双原子造币分子(AuAg,AuCu和AgCu)。除了某些氢键键合的FH化合物外,reg键二元配合物更稳定。AuAg和AuCu分子显示出较大的偶极矩,从而削弱了与Au的区域键(RB),并分别通过Ag和Cu原子形成了偶极矩。
更新日期:2020-09-06
中文翻译:

在双原子造币分子和路易斯酸/碱中建立的区域和氢键之间的竞争。
对由Ag 2和Cu 2与不同分子XH(FH,ClH,OH 2,SH 2,HCN,HNC,HCCH,NH 3和PH 3)形成的可作为氢键供体的配合物的理论研究(路易斯酸)或区域键受体(路易斯碱)是在CCSD(T)/ CBS计算级别上进行的。还考虑了异核双原子造币分子(AuAg,AuCu和AgCu)。除了某些氢键键合的FH化合物外,reg键二元配合物更稳定。AuAg和AuCu分子显示出较大的偶极矩,从而削弱了与Au的区域键(RB),并分别通过Ag和Cu原子形成了偶极矩。











































 京公网安备 11010802027423号
京公网安备 11010802027423号