Journal of Catalysis ( IF 6.5 ) Pub Date : 2020-09-07 , DOI: 10.1016/j.jcat.2020.08.034 Alex M. Román , Naveen Agrawal , Joseph C. Hasse , Michael J. Janik , J. Will Medlin , Adam Holewinski
|
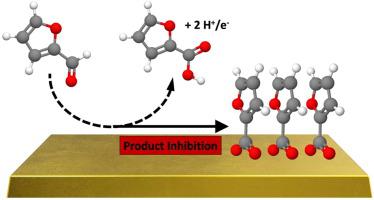
Processing of biomass-derived compounds with electrocatalysis has shown promise to directly couple the production of valuable feedstocks with the storage of renewably produced electricity. One potential route of electrocatalytic conversion is the partial oxidation of furfural to furoic acid (FA), a precursor to 2,5-furandicarboxylic acid (FDCA). We have utilized differential electrochemical reactor studies along with infrared spectroscopy (ATR-SEIRAS) experiments and density functional theory (DFT) calculations to probe the oxidative reaction pathways of furfural on gold catalysts in acidic electrolyte. We find furfural electro-oxidation activity (~2 µA/cm2Au at 1.0 VRHE) to be an order of magnitude higher than that observed on Pt/C. 96 ± 6% Faradaic efficiency to FA is achieved at 0.8 VRHE. Product desorption is rate limiting, and spectroscopic evidence indicates that the most abundant intermediate is surface furoate. Deeper oxidation products observed with dilution of furfural suggest that self-assembly of the furoate species contributes to selectivity.
中文翻译:

糠醛自组装限制了糠醛在金上的电氧化
通过电催化处理生物质衍生的化合物已显示出将有价值的原料的生产与可再生发电的存储直接耦合的希望。电催化转化的一种潜在途径是将糠醛部分氧化为糠酸(FA),糠酸是2,5-呋喃二甲酸(FDCA)的前体。我们利用差分电化学反应器研究以及红外光谱(ATR-SEIRAS)实验和密度泛函理论(DFT)计算来探索糠醛在酸性电解质中金催化剂上的氧化反应途径。我们发现糠醛的电氧化活性(RHE为1.0 V时〜2 µA / cm 2 Au)比在Pt / C上观察到的高一个数量级。在0.8 V RHE时,达到FA的96±6%法拉第效率。产物解吸是限速的,光谱证据表明,最丰富的中间体是表面糠酸酯。糠醛稀释可观察到更深的氧化产物,表明糠酸酯物种的自组装有助于选择性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号