Biochimica et Biophysica Acta (BBA) - Molecular Cell Research ( IF 5.1 ) Pub Date : 2020-09-07 , DOI: 10.1016/j.bbamcr.2020.118846 Alaa Al-Hashimi 1 , Vaishnavi Venugopalan 1 , Naphannop Sereesongsaeng 2 , Sofia Tedelind 1 , Alexandra M Pinzaru 1 , Zeynep Hein 1 , Sebastian Springer 1 , Ekkehard Weber 3 , Dagmar Führer 4 , Christopher J Scott 5 , Roberta E Burden 2 , Klaudia Brix 1
|
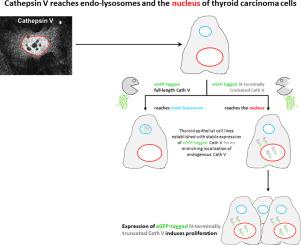
Altered expression and/or localization of cysteine cathepsins is believed to involve in thyroid diseases including cancer. Here, we examined the localization of cathepsins B and V in human thyroid tissue sections of different pathological conditions by immunolabeling and morphometry. Cathepsin B was mostly found within endo-lysosomes as expected. In contrast, cathepsin V was detected within nuclei, predominantly in cells of cold nodules, follicular and papillary thyroid carcinoma tissue, while it was less often detected in this unusual localization in hot nodules and goiter tissue. To understand the significance of nuclear cathepsin V in thyroid cells, this study aimed to establish a cellular model of stable nuclear cathepsin V expression. As representative of a specific form lacking the signal peptide and part of the propeptide, N-terminally truncated cathepsin V fused to eGFP recapitulated the nuclear localization of endogenous cathepsin V throughout the cell cycle in Nthy-ori 3-1 cells. Interestingly, the N-terminally truncated cathepsin V-eGFP was more abundant in the nuclei during S phase. These findings suggested a possible contribution of nuclear cathepsin V forms to cell cycle progression. Indeed, we found that N-terminally truncated cathepsin V-eGFP expressing cells were more proliferative than those expressing full-length cathepsin V-eGFP or wild type controls. We conclude that a specific molecular form of cathepsin V localizes to the nucleus of thyroid epithelial and carcinoma cells, where it might involve in deregulated pathways leading to hyperproliferation. These findings highlight the necessity to better understand cathepsin trafficking in health and disease. In particular, cell type specificity of mislocalization of cysteine cathepsins, which otherwise act in a functionally redundant manner, seems to be important to understand their non-canonical roles in cell cycle progression.
中文翻译:

核组织蛋白酶V在正常甲状腺上皮和癌细胞中的意义。
半胱氨酸组织蛋白酶的改变的表达和/或定位被认为与包括癌症在内的甲状腺疾病有关。在这里,我们通过免疫标记和形态计量学检查了组织蛋白酶B和V在不同病理状况的人甲状腺组织切片中的定位。如所期望的,组织蛋白酶B主要在内溶酶体内发现。相比之下,组织蛋白酶V在细胞核内检测到,主要在冷结节,滤泡状和乳头状甲状腺癌组织的细胞中检测到,而在热结节和甲状腺肿组织的这种异常定位中很少检测到。为了了解核组织蛋白酶V在甲状腺细胞中的重要性,本研究旨在建立稳定的核组织蛋白酶V表达的细胞模型。作为缺少信号肽和部分前肽的特定形式的代表,与eGFP融合的N端截短的组织蛋白酶V在Nthy-ori 3-1细胞的整个细胞周期中概括了内源性组织蛋白酶V的核定位。有趣的是,在S期,N末端截短的组织蛋白酶V-eGFP在细胞核中更为丰富。这些发现表明核组织蛋白酶V形式可能对细胞周期进程的贡献。实际上,我们发现表达N末端截短的组织蛋白酶V-eGFP的细胞比表达全长组织蛋白酶V-eGFP或野生型对照的细胞更增殖。我们得出结论,组织蛋白酶V的特定分子形式位于甲状腺上皮和癌细胞的细胞核中,在这里它可能参与导致过度增殖的失控通路。这些发现强调了更好地了解组织蛋白酶在健康和疾病中的运输的必要性。特别地,半胱氨酸组织蛋白酶错位的细胞类型特异性,否则以功能上多余的方式起作用,对于理解它们在细胞周期进程中的非经典作用似乎很重要。



























 京公网安备 11010802027423号
京公网安备 11010802027423号