Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2020-09-07 , DOI: 10.1016/j.apcatb.2020.119544 Yaxin Yu , Wei Tan , Dongqi An , Xiuwen Wang , Annai Liu , Weixin Zou , Changjin Tang , Chengyan Ge , Qing Tong , Jingfang Sun , Lin Dong
|
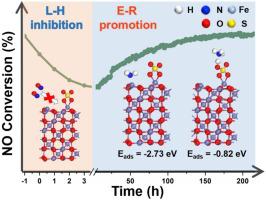
SO2 poisoning of NH3-SCR catalysts at low temperature (< 300 °C) is still an austere challenge. In this work, γ-Fe2O3 was taken as a model catalyst and the effect of reaction temperature on the catalytic activity in the presence of SO2 were fully revealed. SO2 introduction has no negative effect on the activity at 300 °C, which gradually improves with the extension of time. While for 225−275 °C, the activity decreases firstly and then increases slowly. The formatted sulfate species inhibits the adsorption of NOx, cuts off L-H reaction pathway and leads to the initial decline. While the deposited ammonium bisulfate (ABS) can be consumed continuously by NO + O2, implying the formation and consumption of ABS have reached a dynamic equilibrium. Moreover, the formation of ferric sulfate species results in the enhancement of surface acidity, which leads to the promotion of the E-R reaction pathway and further facilitates the increase of activity.
中文翻译:

洞察SO 2导通电阻机制了γ-Fe 2 ö 3在NH催化剂3 -SCR反应:一个合作的实验和研究DFT
在低温(<300°C)下NH 3 -SCR催化剂的SO 2中毒仍然是严峻的挑战。在这项工作中,γ-的Fe 2 ö 3被看作是一个模型的催化剂和反应温度对SO的存在下的催化活性的作用2进行了充分显现。SO 2的引入对300°C的活性没有负面影响,随着时间的延长逐渐增加。在225-275°C时,活性先下降,然后缓慢上升。格式化的硫酸盐物种抑制NO的吸附X,切断了LH反应途径并导致了最初的下降。尽管沉积的硫酸氢铵(ABS)可以被NO + O 2连续消耗,但这意味着ABS的形成和消耗已达到动态平衡。而且,硫酸铁物质的形成导致表面酸度的提高,这导致ER反应途径的促进并且进一步促进了活性的增加。











































 京公网安备 11010802027423号
京公网安备 11010802027423号