Reactive & Functional Polymers ( IF 4.5 ) Pub Date : 2020-09-05 , DOI: 10.1016/j.reactfunctpolym.2020.104720 Jiahong Wang , Min Mao , Saleem Atif , Yao Chen
|
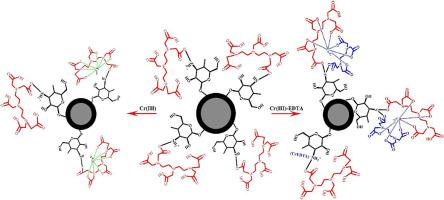
The presence of widely used chelating agents such as ethylenediaminetetraacetic acid (EDTA) in aqueous solution increases the difficulty of heavy metal removal by the formation of heavy metal chelates. In this study, diethylenetriaminepentaacetic acid (DTPA)-chitosan functionalized magnetic silica (Fe3O4@SiO2/Cs-DTPA) was developed for the removal of Cr(III) and Cr(III)-EDTA. The obtained results from characterization confirmed that Fe3O4@SiO2/Cs-DTPA with the core-shell structure and superparamagnetic properties was synthesized. The maximum theoretical adsorption capacity of Cr(III) and Cr(III)-EDTA chelates on the adsorbents were 39.27 and 22.24 mg/g at 25 °C and pH 4.0, respectively. Adsorption equilibrium of Cr(III) and Cr(III)-EDTA on Fe3O4@SiO2/Cs-DTPA was achieved within 15 min, and the adsorption data fit the pseudo second-order model. The co-existing complex agents (EDTA) and cations (Na+, Ca2+, and Mg2+) in the aqueous solution reduced Cr(III) adsorption by the competition with Cr(III) and Cr(III)-EDTA chelate for active groups of the adsorbent. The affinity of the Fe3O4@SiO2/Cs-DTPA for Cr(III) and Cr(III)-EDTA was excellent even at high salt and organic chelates concentration. The Cr(III) adsorption was attributed to surface complexation between Cr(III) and DTPA moieties of the adsorbent, while Cr(III)-EDTA as a whole was adsorbed on the adsorbent by the complexation and electrostatic interaction. Cr(III) saturated Fe3O4@SiO2/Cs-DTPA can be readily regenerated in acid solution and Cr(III) or Cr(III)-EDTA adsorption of regenerated adsorbent exhibits no obvious reduce.
中文翻译:

DTPA-壳聚糖修饰的Fe 3 O 4 @SiO 2对Cr(III)和Cr(III)-EDTA螯合物的吸附行为及其机理
水溶液中广泛使用的螯合剂(例如乙二胺四乙酸(EDTA))的存在增加了通过形成重金属螯合物去除重金属的难度。在这项研究中,开发了二亚乙基三胺五乙酸(DTPA)-壳聚糖功能化的磁性二氧化硅(Fe 3 O 4 @SiO 2 / Cs-DTPA),用于去除Cr(III)和Cr(III)-EDTA。通过表征获得的结果证实Fe 3 O 4 @SiO 2合成了具有核壳结构和超顺磁性的/ Cs-DTPA。在25°C和pH 4.0下,Cr(III)和Cr(III)-EDTA螯合物在吸附剂上的最大理论吸附容量分别为39.27和22.24 mg / g。Cr(III)和Cr(III)-EDTA在Fe 3 O 4 @SiO 2 / Cs-DTPA上的吸附平衡达到15分钟,吸附数据符合拟二阶模型。水溶液中共存的络合剂(EDTA)和阳离子(Na +,Ca 2+和Mg 2+)通过与Cr(III)和Cr(III)-EDTA螯合物竞争降低了Cr(III)的吸附。用于吸附剂的活性基团。Fe 3 O 4的亲和力即使在高盐和有机螯合物浓度下,Cr(III)和Cr(III)-EDTA的@SiO 2 / Cs-DTPA也非常好。Cr(III)的吸附归因于Cr(III)与DTPA部分之间的表面络合,而Cr(III)-EDTA则通过络合和静电相互作用整体吸附在吸附剂上。Cr(III)饱和Fe 3 O 4 @SiO 2 / Cs-DTPA可以在酸性溶液中容易地再生,并且再生吸附剂的Cr(III)或Cr(III)-EDTA吸附没有明显降低。









































 京公网安备 11010802027423号
京公网安备 11010802027423号