当前位置:
X-MOL 学术
›
J. Phys. Chem. Solids
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
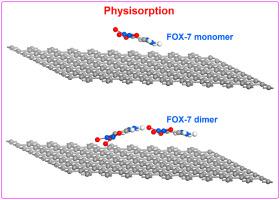
Noncovalent functionalization of graphene through physisorption of 1,1-diamino-2,2-dinitroethene: Impacts of and cooperativity between hydrogen bond and π···π interaction
Journal of Physics and Chemistry of Solids ( IF 4.3 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.jpcs.2020.109736 Yingzhe Liu , Tao Yu , Weipeng Lai , Yiding Ma , Zhongxue Ge , Fang-Ling Yang , Pan-Pan Zhou , Yong-Hui Zhang , Kefeng Xie
Journal of Physics and Chemistry of Solids ( IF 4.3 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.jpcs.2020.109736 Yingzhe Liu , Tao Yu , Weipeng Lai , Yiding Ma , Zhongxue Ge , Fang-Ling Yang , Pan-Pan Zhou , Yong-Hui Zhang , Kefeng Xie
|
Abstract The rich π-electrons region of graphene enables it to be a good material which can interact with many organic molecules. As a low-sensitivity and high-energy explosive, 1,1-diamino-2,2-dinitroethene and its dimer possess unique characters including hydrogen bonds and π bond which can adsorb on graphene through physisorption. The results suggest that the adsorption energies have a very small difference on the different adsorption sites. Due to the physisorption, the geometrical and electronic properties of 1,1-diamino-2,2-dinitroethene and its dimer vary significantly. The intramolecular as well as intermolecular hydrogen bond strengths are considerably affected due to the π···π interaction of 1,1-diamino-2,2-dinitroethene or its dimer with graphene. The cooperativity between intermolecular hydrogen bonds and π···π interactions is observed which causes wax of intermolecular hydrogen bonds and wane of π···π interactions. The physisorption of 1,1-diamino-2,2-dinitroethene or its dimer also leads to the variation of electronic property of graphene.
中文翻译:

通过物理吸附 1,1-二氨基-2,2-二硝基乙烯对石墨烯的非共价功能化:氢键与 π···π 相互作用的影响和协同作用
摘要 石墨烯丰富的π电子区使其成为一种可以与多种有机分子相互作用的优良材料。1,1-二氨基-2,2-二硝基乙烯及其二聚体作为一种低敏高能炸药,具有氢键和π键等独特特性,可通过物理吸附作用吸附在石墨烯上。结果表明,不同吸附位点的吸附能差异很小。由于物理吸附,1,1-二氨基-2,2-二硝基乙烯及其二聚体的几何和电子特性变化很大。由于1,1-二氨基-2,2-二硝基乙烯或其二聚体与石墨烯的π…π相互作用,分子内和分子间氢键强度受到显着影响。观察到分子间氢键与π···π相互作用之间的协同作用,导致分子间氢键蜡化和π···π相互作用减弱。1,1-二氨基-2,2-二硝基乙烯或其二聚体的物理吸附也导致石墨烯电子性质的变化。
更新日期:2021-01-01
中文翻译:

通过物理吸附 1,1-二氨基-2,2-二硝基乙烯对石墨烯的非共价功能化:氢键与 π···π 相互作用的影响和协同作用
摘要 石墨烯丰富的π电子区使其成为一种可以与多种有机分子相互作用的优良材料。1,1-二氨基-2,2-二硝基乙烯及其二聚体作为一种低敏高能炸药,具有氢键和π键等独特特性,可通过物理吸附作用吸附在石墨烯上。结果表明,不同吸附位点的吸附能差异很小。由于物理吸附,1,1-二氨基-2,2-二硝基乙烯及其二聚体的几何和电子特性变化很大。由于1,1-二氨基-2,2-二硝基乙烯或其二聚体与石墨烯的π…π相互作用,分子内和分子间氢键强度受到显着影响。观察到分子间氢键与π···π相互作用之间的协同作用,导致分子间氢键蜡化和π···π相互作用减弱。1,1-二氨基-2,2-二硝基乙烯或其二聚体的物理吸附也导致石墨烯电子性质的变化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号