当前位置:
X-MOL 学术
›
Magn. Reson. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Monitoring of the sedimentation kinetics of vaccine adjuvants using water proton NMR relaxation
Magnetic Resonance in Chemistry ( IF 1.9 ) Pub Date : 2020-09-16 , DOI: 10.1002/mrc.5096 Marc B Taraban 1, 2 , Yihua Bruce Yu 1, 2
Magnetic Resonance in Chemistry ( IF 1.9 ) Pub Date : 2020-09-16 , DOI: 10.1002/mrc.5096 Marc B Taraban 1, 2 , Yihua Bruce Yu 1, 2
Affiliation
|
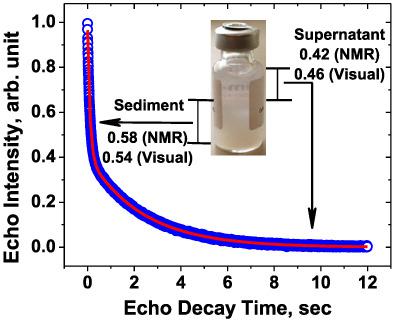
Suspensions of solid particles find applications in many areas-mining, waste treatment, and in pharmaceutical formulations. Pharmaceutical suspensions include aluminum-adjuvanted vaccines widely administered to millions of people worldwide annually. Hence, the stability parameters of such suspensions, e. g., sedimentation rate and the compactness of the formed sediments are of great interest to achieve the most optimal and stable formulations. Unlike currently used analytical techniques involving visual observations and/or monitoring of several optical properties using specialized glassware, water proton NMR (wNMR) used in this work allows one to analyze samples in their original sealed container regardless of its opacity and/or labeling. It was demonstrated that the water proton transverse relaxation rate could be used to monitor in real time the sedimentation process of two widely used aluminum adjuvants- Alhydrogel® and Adju-Phos®. Using wNMR, we obtained valuable information on the sedimentation rate, dynamics of the supernatant and sediment formation, and the sedimentation volume ratio (SVR) reflecting the compactness of the formed sediment. Results on SVR from wNMR were verified by caliper measurements. Verification of the sedimentation rate results from wNMR by other analytical techniques is challenging due to differences in the measured attributes and even units of the reported rate. Nonetheless, our results demonstrate the practical applicability of wNMR as an analytical tool to study pharmaceutical suspensions, e. g., aluminum-adjuvanted vaccines, to provide higher quality and more efficient vaccines. Such analyses could be carried out in the original container of a suspension drug product to study its colloidal stability and to monitor its quality over time without compromising product integrity.
中文翻译:

使用水质子核磁共振弛豫监测疫苗佐剂的沉降动力学
固体颗粒的悬浮液在许多领域都有应用——采矿、废物处理和药物制剂。药物混悬液包括铝佐剂疫苗,每年广泛应用于全球数百万人。因此,此类悬浮液的稳定性参数,例如沉降速率和形成的沉积物的密实度,对于获得最优化和最稳定的配方非常重要。与目前使用的涉及使用专用玻璃器皿进行视觉观察和/或监测几种光学特性的分析技术不同,这项工作中使用的水质子核磁共振 (wNMR) 允许人们分析其原始密封容器中的样品,而不管其不透明度和/或标签如何。结果表明,水质子横向弛豫率可用于实时监测两种广泛使用的铝佐剂——Alhydrogel® 和 Adju-Phos® 的沉降过程。使用 wNMR,我们获得了有关沉降速率、上清液和沉积物形成动力学以及反映所形成沉积物致密性的沉降体积比 (SVR) 的宝贵信息。来自 wNMR 的 SVR 结果通过卡尺测量得到验证。由于测量属性甚至报告速率的单位存在差异,因此通过其他分析技术验证来自 wNMR 的沉降速率结果具有挑战性。尽管如此,我们的结果证明了 wNMR 作为研究药物悬浮液的分析工具的实际适用性,例如铝佐剂疫苗,提供更高质量和更有效的疫苗。此类分析可以在悬浮药物产品的原始容器中进行,以研究其胶体稳定性并随着时间的推移监测其质量,而不会影响产品的完整性。
更新日期:2020-09-16
中文翻译:

使用水质子核磁共振弛豫监测疫苗佐剂的沉降动力学
固体颗粒的悬浮液在许多领域都有应用——采矿、废物处理和药物制剂。药物混悬液包括铝佐剂疫苗,每年广泛应用于全球数百万人。因此,此类悬浮液的稳定性参数,例如沉降速率和形成的沉积物的密实度,对于获得最优化和最稳定的配方非常重要。与目前使用的涉及使用专用玻璃器皿进行视觉观察和/或监测几种光学特性的分析技术不同,这项工作中使用的水质子核磁共振 (wNMR) 允许人们分析其原始密封容器中的样品,而不管其不透明度和/或标签如何。结果表明,水质子横向弛豫率可用于实时监测两种广泛使用的铝佐剂——Alhydrogel® 和 Adju-Phos® 的沉降过程。使用 wNMR,我们获得了有关沉降速率、上清液和沉积物形成动力学以及反映所形成沉积物致密性的沉降体积比 (SVR) 的宝贵信息。来自 wNMR 的 SVR 结果通过卡尺测量得到验证。由于测量属性甚至报告速率的单位存在差异,因此通过其他分析技术验证来自 wNMR 的沉降速率结果具有挑战性。尽管如此,我们的结果证明了 wNMR 作为研究药物悬浮液的分析工具的实际适用性,例如铝佐剂疫苗,提供更高质量和更有效的疫苗。此类分析可以在悬浮药物产品的原始容器中进行,以研究其胶体稳定性并随着时间的推移监测其质量,而不会影响产品的完整性。










































 京公网安备 11010802027423号
京公网安备 11010802027423号