当前位置:
X-MOL 学术
›
Chem. Bio. Drug Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, synthesis and anti‐inflammatory study of novel N‐heterocyclic substituted Aloe‐emodin derivatives
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2020-09-05 , DOI: 10.1111/cbdd.13788 Xiang Qiu 1 , Heying Pei 2 , Hengfan Ni 1 , Zhengying Su 2 , Yong Li 2 , Zhuang Yang 2 , Caixia Dou 1 , Lijuan Chen 2 , Li Wan 1
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2020-09-05 , DOI: 10.1111/cbdd.13788 Xiang Qiu 1 , Heying Pei 2 , Hengfan Ni 1 , Zhengying Su 2 , Yong Li 2 , Zhuang Yang 2 , Caixia Dou 1 , Lijuan Chen 2 , Li Wan 1
Affiliation
|
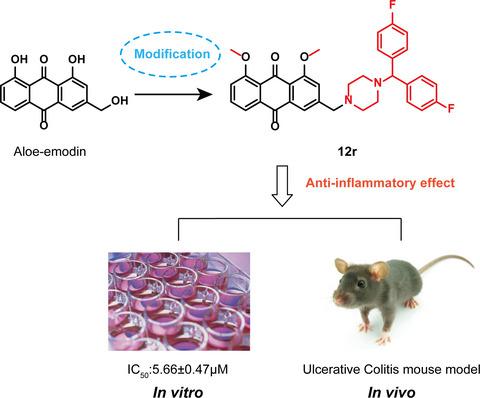
A novel series of Aloe‐emodin derivatives containing N‐heterocyclic moieties was designed and synthesized. The structure‐activity relationship studies (SARs) indicated that the replacement of hydroxyethyl and benzhydryl piperazine groups could improve efficacy. Compounds 12r and 14a–14c exhibited a higher inhibitory effect on LPS‐induced nitric oxide (NO) production in RAW264.7 macrophages than Aloe‐emodin did. Among them, 12r showed the most potent inhibition with an IC50 value of 5.66 ± 0.47 μM. Further toxicity and pharmacokinetic studies were carried out and 12r was found to be the most active structure with low toxicity risk and good metabolic properties. It could also decrease the levels of IL‐1β, TNF‐α, PGE2 and inhibit the activation of nuclear factor‐κB signalling pathway. Importantly, 12r showed oral bioavailability of up to 55.16% and attenuated the inflammatory symptoms in an ulcerative colitis mouse model in vivo. These results indicate that 12r is suitable for development as an anti‐inflammatory agent.
中文翻译:

新型 N-杂环取代芦荟大黄素衍生物的设计、合成和抗炎研究
设计并合成了一系列含有 N-杂环部分的新型芦荟大黄素衍生物。构效关系研究(SARs)表明,取代羟乙基和二苯甲基哌嗪基团可以提高疗效。与芦荟大黄素相比,化合物12r和14a - 14c对 RAW264.7 巨噬细胞中 LPS 诱导的一氧化氮 (NO) 产生具有更高的抑制作用。其中,12r显示出最有效的抑制作用,IC 50值为 5.66 ± 0.47 μM。进行了进一步的毒性和药代动力学研究,12r被发现是最活跃的结构,具有低毒性风险和良好的代谢特性。它还可以降低IL-1β、TNF-α、PGE 2 的水平并抑制核因子-κB信号通路的激活。重要的是,12r 的口服生物利用度高达 55.16%,并在体内溃疡性结肠炎小鼠模型中减轻了炎症症状。这些结果表明12r适合作为抗炎剂开发。
更新日期:2020-09-05
中文翻译:

新型 N-杂环取代芦荟大黄素衍生物的设计、合成和抗炎研究
设计并合成了一系列含有 N-杂环部分的新型芦荟大黄素衍生物。构效关系研究(SARs)表明,取代羟乙基和二苯甲基哌嗪基团可以提高疗效。与芦荟大黄素相比,化合物12r和14a - 14c对 RAW264.7 巨噬细胞中 LPS 诱导的一氧化氮 (NO) 产生具有更高的抑制作用。其中,12r显示出最有效的抑制作用,IC 50值为 5.66 ± 0.47 μM。进行了进一步的毒性和药代动力学研究,12r被发现是最活跃的结构,具有低毒性风险和良好的代谢特性。它还可以降低IL-1β、TNF-α、PGE 2 的水平并抑制核因子-κB信号通路的激活。重要的是,12r 的口服生物利用度高达 55.16%,并在体内溃疡性结肠炎小鼠模型中减轻了炎症症状。这些结果表明12r适合作为抗炎剂开发。











































 京公网安备 11010802027423号
京公网安备 11010802027423号