Journal of King Saud University-Science ( IF 3.7 ) Pub Date : 2020-09-05 , DOI: 10.1016/j.jksus.2020.09.001 Abdulrahman I. Almansour , Raju Suresh Kumar , Natarajan Arumugam
|
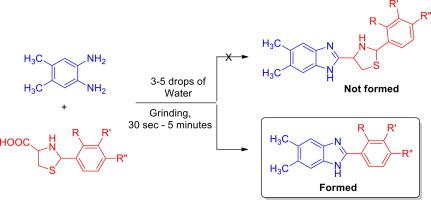
A versatile green chemical procedure for the highly selective construction of 2-aryl substituted benz-/naphthimidazoles starting from the reaction of aromatic 1,2-diamines with a series of substituted arylthioprolines with three to five drops of water under simple grinding at ambient temperature in good yields is described. The short reaction time, simplified experimental procedure, the absence of extraction and chromatographic purification steps in addition to the environment affability makes this green strategy highly attractive in view of green chemistry. The expected reaction to furnish the thiazole grafted benz-/naphthimidazole did not occur. Perhaps the arylthioprolines could be in zwitterionic form, which could react with 1,2-diamine giving dihyrobenzimidazole, which undergoes air oxidation to furnish the 2-aryl benzimidazole rather than the expected thiazole grafted imidazoles.
中文翻译:

一种简单,快速,方便且可持续的绿色策略,用于合成苯并/萘并咪唑
在环境温度下简单研磨下,由芳族1,2-二胺与一系列取代的芳基硫代脯氨酸与三至五滴水的反应开始,可高度选择性地构建2-芳基取代的苯并/萘二咪唑的通用绿色化学程序。描述了良好的产量。短的反应时间,简化的实验程序,除了具有环境亲和力外,没有萃取和色谱纯化步骤,因此从绿色化学的角度来看,这种绿色策略极具吸引力。没有发生提供噻唑接枝的苯并/萘并咪唑的预期反应。芳硫基脯氨酸可能是两性离子形式,可以与1,2-二胺反应生成二氢苯并咪唑,









































 京公网安备 11010802027423号
京公网安备 11010802027423号