当前位置:
X-MOL 学术
›
J. Chem. Thermodyn.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thermodynamic studies of aggregation behaviour of cationic surfactants (octyltrimethylammonium chloride / tetradecyltrimethylammonium chloride) in aqueous solutions at different temperatures
The Journal of Chemical Thermodynamics ( IF 2.2 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.jct.2020.106282 Vasim R. Shaikh , A. Abdul , Kesharsingh J. Patil
The Journal of Chemical Thermodynamics ( IF 2.2 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.jct.2020.106282 Vasim R. Shaikh , A. Abdul , Kesharsingh J. Patil
|
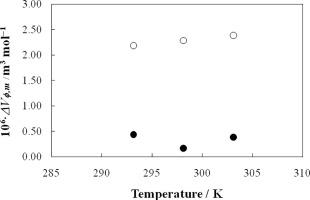
Abstract Densities for aqueous solutions of cationic surfactants, namely, octyltrimethylammonium chloride (C8TAC) and tetradecyltrimethylammonium chloride (C14TAC) in the concentration range (0.0102–0.2599) mol⋅kg−1 have been measured at (293.15, 298.15 and 303.15) K. Further, experimental density data are utilized for the calculations of apparent molar volumes of solute ( V ϕ ) at studied temperatures. The coefficient of thermal expansion (α) and apparent molar expansivity ( E ϕ ) are also obtained at 298.15 K. Application of pseudo-phase model of micellization to the V ϕ data is made for obtaining critical micelle concentration (cmc) of the studied surfactants. The magnitudes of cmc values are in the order of C8TAC > C14TAC. The change in the apparent molar volume due to aggregation ( Δ V ϕ , m ) is found to be dependent on temperature as well on the chain length of the alkyltrimethlyammoinium ions. Δ V ϕ , m slightly increases with increase in temperature and in chain length of the alkyltrimethlyammoinium ions. The standard free energy change due to micellization ( Δ G m 0 ) have also been estimated. All the results are explained on the basis of micellization of the surfactant molecules, hydrophobicity due to alkyl chain length and nature of the counter-ion.
中文翻译:

阳离子表面活性剂(辛基三甲基氯化铵/十四烷基三甲基氯化铵)在不同温度水溶液中聚集行为的热力学研究
摘要 阳离子表面活性剂水溶液的密度,即辛基三甲基氯化铵 (C8TAC) 和十四烷基三甲基氯化铵 (C14TAC) 在 (0.0102–0.2599) mol·kg−1 浓度范围内的密度已在 (293.15, 298.15 和 303.15) 处测得。 ,实验密度数据用于计算所研究温度下溶质的表观摩尔体积 (V ϕ )。热膨胀系数 (α) 和表观摩尔膨胀系数 ( E ϕ ) 也在 298.15 K 处获得。 将胶束化假相模型应用于 V ϕ 数据以获得所研究表面活性剂的临界胶束浓度 (cmc) . cmc 值的大小顺序为 C8TAC > C14TAC。由于聚集引起的表观摩尔体积的变化 ( Δ V ϕ , 发现 m ) 取决于温度以及烷基三甲基氨鎓离子的链长。Δ V ϕ , m 随着温度的增加和烷基三甲基铵离子的链长的增加而略微增加。还估计了由于胶束化引起的标准自由能变化 (Δ G m 0 )。所有结果都基于表面活性剂分子的胶束化、由于烷基链长度和反离子的性质而产生的疏水性。
更新日期:2021-01-01
中文翻译:

阳离子表面活性剂(辛基三甲基氯化铵/十四烷基三甲基氯化铵)在不同温度水溶液中聚集行为的热力学研究
摘要 阳离子表面活性剂水溶液的密度,即辛基三甲基氯化铵 (C8TAC) 和十四烷基三甲基氯化铵 (C14TAC) 在 (0.0102–0.2599) mol·kg−1 浓度范围内的密度已在 (293.15, 298.15 和 303.15) 处测得。 ,实验密度数据用于计算所研究温度下溶质的表观摩尔体积 (V ϕ )。热膨胀系数 (α) 和表观摩尔膨胀系数 ( E ϕ ) 也在 298.15 K 处获得。 将胶束化假相模型应用于 V ϕ 数据以获得所研究表面活性剂的临界胶束浓度 (cmc) . cmc 值的大小顺序为 C8TAC > C14TAC。由于聚集引起的表观摩尔体积的变化 ( Δ V ϕ , 发现 m ) 取决于温度以及烷基三甲基氨鎓离子的链长。Δ V ϕ , m 随着温度的增加和烷基三甲基铵离子的链长的增加而略微增加。还估计了由于胶束化引起的标准自由能变化 (Δ G m 0 )。所有结果都基于表面活性剂分子的胶束化、由于烷基链长度和反离子的性质而产生的疏水性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号