当前位置:
X-MOL 学术
›
J. Chem. Thermodyn.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Solubility determination and thermodynamic modelling of solid−liquid equilibria in the (NaCl + NaBO2 + Na2B4O7 + H2O) system at 298.15 K
The Journal of Chemical Thermodynamics ( IF 2.6 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.jct.2020.106283 Lele Chen , Xiuxiu Yang , Yafei Guo , Tianlong Deng , Dan Li , Lingzong Meng
The Journal of Chemical Thermodynamics ( IF 2.6 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.jct.2020.106283 Lele Chen , Xiuxiu Yang , Yafei Guo , Tianlong Deng , Dan Li , Lingzong Meng
|
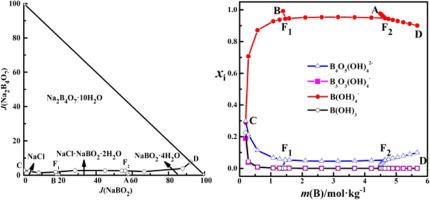
Abstract The solubility corresponding solution physicochemical properties (densities, refractive indices and pH) in the quaternary system (NaCl + NaBO2 + Na2B4O7 + H2O) were investigated at 298.15 K by the isothermal dissolution equilibrium method. The dry-salt phase diagram, water content diagram, refractive index diagram and pH diagram were drawn. There are four crystallization fields corresponding to NaCl, NaBO2·4H2O, Na2B4O7·10H2O, NaCl·NaBO2·2H2O, five univariant curves and two invariant points in the phase diagram of the quaternary system. The solubility, concentrations of boron species B(OH)3, B(OH)4−, B3O3(OH)4−, B4O5(OH)42− and OH− in the system were calculated using the Pitzer model. The variation trends for calculated solubility curves and pH curves are consistent with the experimental results. The distributions of different boron species were obtained in the mixed borate solution. The distributions of four boron species were obtained in the mixed borate solution. The main boron species in the mixed borate solution are B(OH)4− and B4O5(OH)42−. The mole fractions of the four boron species are mainly affected by m(B) in the solution but less by m(Cl−).
中文翻译:

(NaCl + NaBO2 + Na2B4O7 + H2O) 系统中固液平衡的溶解度测定和热力学建模在 298.15 K
摘要 采用等温溶解平衡法研究了在298.15 K下四元体系(NaCl + NaBO2 + Na2B4O7 + H2O)中溶解度对应的溶液理化性质(密度、折射率和pH)。绘制了干盐相图、含水量图、折光率图和pH图。在四元系相图中,有四个对应于NaCl、NaBO2·4H2O、Na2B4O7·10H2O、NaCl·NaBO2·2H2O的结晶场,5条单变曲线和2个不变点。使用 Pitzer 模型计算系统中硼物质 B(OH)3、B(OH)4-、B3O3(OH)4-、B4O5(OH)42- 和 OH- 的溶解度、浓度。计算出的溶解度曲线和pH曲线的变化趋势与实验结果一致。在混合硼酸盐溶液中获得了不同硼种类的分布。在混合硼酸盐溶液中获得了四种硼物质的分布。混合硼酸盐溶液中的主要硼种类是 B(OH)4- 和 B4O5(OH)42-。四种硼物质的摩尔分数主要受溶液中 m(B) 的影响,但受 m(Cl−) 影响较小。
更新日期:2021-01-01
中文翻译:

(NaCl + NaBO2 + Na2B4O7 + H2O) 系统中固液平衡的溶解度测定和热力学建模在 298.15 K
摘要 采用等温溶解平衡法研究了在298.15 K下四元体系(NaCl + NaBO2 + Na2B4O7 + H2O)中溶解度对应的溶液理化性质(密度、折射率和pH)。绘制了干盐相图、含水量图、折光率图和pH图。在四元系相图中,有四个对应于NaCl、NaBO2·4H2O、Na2B4O7·10H2O、NaCl·NaBO2·2H2O的结晶场,5条单变曲线和2个不变点。使用 Pitzer 模型计算系统中硼物质 B(OH)3、B(OH)4-、B3O3(OH)4-、B4O5(OH)42- 和 OH- 的溶解度、浓度。计算出的溶解度曲线和pH曲线的变化趋势与实验结果一致。在混合硼酸盐溶液中获得了不同硼种类的分布。在混合硼酸盐溶液中获得了四种硼物质的分布。混合硼酸盐溶液中的主要硼种类是 B(OH)4- 和 B4O5(OH)42-。四种硼物质的摩尔分数主要受溶液中 m(B) 的影响,但受 m(Cl−) 影响较小。



























 京公网安备 11010802027423号
京公网安备 11010802027423号