Tetrahedron ( IF 2.1 ) Pub Date : 2020-09-06 , DOI: 10.1016/j.tet.2020.131567 Yi Zhu , Li Ge , Yuxin Chen , Yande Chen , Yu Liang , Yidan Wang , Kedi Yang
|
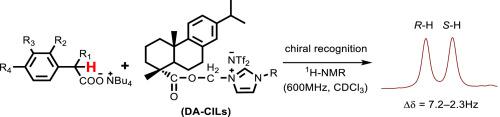
Several new chiral imidazolinium ionic liquids (CILs) have been synthesized by using optically pure dehydroabietic acid (DA) as the chiral precursor. Their enantiomeric recognition abilities were measured using rac-mandelic acid n-Bu4N salt as the substrate by NMR spectroscopy. Based on the structure-interaction relationships, the bulky anion and the side chain linking the DA group to the imidazole ring have crucial effects on CIL recognition. The 1H NMR measurements indicated that [MimMeDA]NTf2 exhibits good and versatile molecular recognition ability. The new DA-based CILs reported here may be interesting chiral media for enantioselective reactions and useful in enantiomeric separations.
中文翻译:

基于脱氢松香酸的手性离子液体:它们的合成和潜在的对映体识别能力
通过使用光学纯的脱氢松香酸(DA)作为手性前体,合成了几种新型手性咪唑啉鎓离子液体(CIL)。通过rac-扁桃酸n- Bu 4 N盐作为底物通过NMR光谱法测量它们的对映体识别能力。基于结构-相互作用关系,大阴离子和连接DA基团与咪唑环的侧链对CIL识别具有关键作用。的1个1 H NMR测量表明,[MimMeDA] NTF 2显示出良好的和通用的分子识别能力。此处报道的新的基于DA的新CIL可能是用于对映选择性反应的有趣手性介质,可用于对映体分离。



























 京公网安备 11010802027423号
京公网安备 11010802027423号