Tetrahedron ( IF 2.1 ) Pub Date : 2020-09-06 , DOI: 10.1016/j.tet.2020.131570 Faisal Jamshaid , Vishnu V.R. Kondakal , C. Declan Newman , Rhianne Dobson , Heidi João , Craig R. Rice , Joseph M. Mwansa , Bimod Thapa , Karl Hemming
|
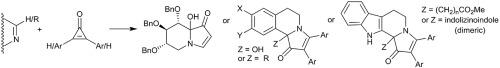
An attempted synthesis of the indolizidine natural product castanospermine resulted in the successful addition of cyclopropenone to a sugar-derived poly-hydroxylated cyclic imine to give an indolizidinone product, but with the installation of an extra hydroxy group at the castanospermine 8a-bridgehead position. This was also observed in our previous approach to the australine and hyacinthacine pyrrolizidine natural products. The same oxidative phenomenon occurred during the synthesis of pyrrolo[1,2-a]isoquinolines from the reaction of aldimine dihydroisoquinolines with cyclopropenones, whereas ketimine based dihydroisoquinolines gave pyrrolo[1,2-a]isoquinolines without bridgehead oxidation. These results may have some significance for the origins of the bridgehead hydroxy natural products jenamidine B1/B2, clazamycin A/B and legonmycin A/B. The precursor cyclic aldimine for the synthesis of the indolizino[8,7-b]indoles gave dimeric indolizino[8,7-b]indoles, whereas the corresponding cyclic ketimines behaved as expected and gave the indolizino[8,7-b]indole core after reaction with cyclopropenones.
中文翻译:

在吲哚里,吡咯并[2,1-的合成Cyclopropenones一个]异喹啉和吲嗪并[8,7- b ]吲哚生物碱
吲哚并咪唑天然产物粟精胺的尝试合成导致成功地将环丙烯酮添加到糖衍生的多羟基化环亚胺中,得到吲哚嗪酮产品,但是在粟精胺8a-桥头位置上安装了额外的羟基。这在我们以前对奥曲林和风信子吡咯并核苷天然产物的处理中也观察到。由醛亚胺二氢异喹啉与环丙烯酮反应合成吡咯并[1,2- a ]异喹啉时发生了相同的氧化现象,而酮亚胺基二氢异喹啉则产生了吡咯并[1,2- a]异喹啉,无桥头氧化。这些结果可能对桥头羟基天然产物啶B 1 / B 2,克拉扎霉素A / B和莱顿霉素A / B的起源具有重要意义。用于合成吲哚并[8,7- b ]吲哚的前体环状醛亚胺产生二聚吲哚并[8,7- b ]吲哚,而相应的环状酮亚胺表现出预期并得到吲哚并[8,7- b ]吲哚。与环丙烯酮反应后的核心。











































 京公网安备 11010802027423号
京公网安备 11010802027423号