Journal of Molecular Biology ( IF 5.6 ) Pub Date : 2020-09-06 , DOI: 10.1016/j.jmb.2020.08.023 Michael P Lockhart-Cairns 1 , Helena Newandee 2 , Jennifer Thomson 1 , Anthony S Weiss 3 , Clair Baldock 1 , Anna Tarakanova 4
|
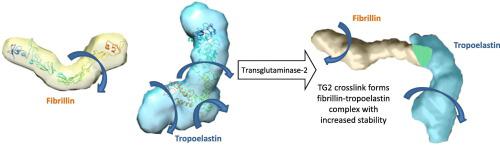
Elastic fibres are essential components of all mammalian elastic tissues such as blood vessels, lung and skin, and are critically important for the mechanical properties they endow. The main components of elastic fibres are elastin and fibrillin, where correct formation of elastic fibres requires a fibrillin microfibril scaffold for the deposition of elastin. It has been demonstrated previously that the interaction between fibrillin and tropoelastin, the elastin precursor, increases the rate of assembly of tropoelastin. Furthermore, tropoelastin and fibrillin can be cross-linked by transglutaminase-2, but the function of cross-linking on their elastic properties is yet to be elucidated. Here we show that transglutaminase cross-linking supports formation of a 1:1 stoichiometric fibrillin–tropoelastin complex. SAXS data show that the complex retains features of the individual proteins but is elongated supporting end-to-end assembly. Elastic network models were constructed to compare the dynamics of tropoelastin and fibrillin individually as well as in the cross-linked complex. Normal mode analysis was performed to determine the structures' most energetically favourable, biologically accessible motions which show that within the complex, tropoelastin is less mobile and this molecular stabilisation extends along the length of the tropoelastin molecule to regions remote from the cross-linking site. Together, these data suggest a long-range stabilising effect of cross-linking that occurs due to the covalent linkage of fibrillin to tropoelastin. This work provides insight into the interactions of tropoelastin and fibrillin and how cross-link formation stabilises the elastin precursor so it is primed for elastic fibre assembly.
中文翻译:

转谷氨酰胺酶介导的原弹性蛋白与原纤维蛋白的交联在弹性纤维组装之前稳定了弹性蛋白前体。
弹性纤维是所有哺乳动物弹性组织(如血管、肺和皮肤)的重要组成部分,对于它们赋予的机械性能至关重要。弹性纤维的主要成分是弹性蛋白和原纤维蛋白,其中弹性纤维的正确形成需要原纤维蛋白微原纤维支架用于弹性蛋白的沉积。先前已经证明,原纤维蛋白与弹性蛋白前体原弹性蛋白之间的相互作用增加了原弹性蛋白的组装速率。此外,原弹性蛋白和原纤维蛋白可以通过转谷氨酰胺酶2进行交联,但交联对其弹性特性的作用尚待阐明。在这里,我们表明转谷氨酰胺酶交联支持 1:1 化学计量的原纤维蛋白 - 原弹性蛋白复合物的形成。SAXS 数据显示,复合物保留了单个蛋白质的特征,但被拉长,支持端到端组装。构建弹性网络模型以比较原弹性蛋白和原纤维蛋白的动力学以及在交联复合物中的动力学。进行正态模式分析以确定结构在能量上最有利的、生物学上可接近的运动,这表明在复合物中,原弹性蛋白的移动性较低,并且这种分子稳定性沿着原弹性蛋白分子的长度延伸到远离交联位点的区域。总之,这些数据表明由于原纤维蛋白与原弹性蛋白的共价连接而发生的交联的长期稳定作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号