Journal of Energy Chemistry ( IF 13.1 ) Pub Date : 2020-09-05 , DOI: 10.1016/j.jechem.2020.08.051 Han Wu , Yaojia Cheng , Boyang Wang , Yao Wang , Min Wu , Weidong Li , Baozhong Liu , Siyu Lu
|
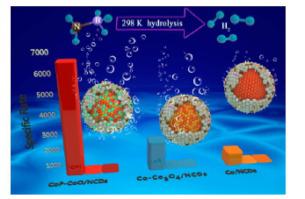
Ammonia borane (NH3BH3, AB) is promising for chemical hydrogen storage; however, current systems for rapid hydrogen production are limited by the expensive noble metal catalysts required for AB hydrolysis. Here we report the design and synthesis of a highly efficient and robust non-noble-metal catalyst for the hydrolysis of AB at 298 K (TOF = 89.56 molH2 min−1 molCo−1). Experiments and density functional theory calculations were performed to explore the catalyst’s hybrid nanoparticle heterostructure and its catalytic mechanism. The catalyst comprised nitrogen-doped carbon dots confining CoO and CoP, and exhibited strong interface-induced synergistic catalysis for AB hydrolysis that effectively decreased the energy barriers for the dissociation of both AB and water molecules. The co-doping of N and P introduced numerous defects, and further regulated the reactivity of the carbon layers. The heterogeneous interface design technique presented here provides a new strategy for developing efficient and inexpensive non-noble-metal catalysts that may be applicable in other fields related to energy catalysis.
中文翻译:

具有强界面协同作用的碳点约束的CoP-CoO纳米异质结构触发了氨硼烷强劲的氢释放
氨硼烷(NH 3 BH 3,AB)有望用于化学储氢;然而,目前快速制氢的系统受到AB水解所需的昂贵贵金属催化剂的限制。在这里,我们报告了一种高效,稳健的非贵金属催化剂的设计和合成,该催化剂可在298 K下水解AB(TOF = 89.56 mol H2 min -1 mol Co -1)。进行了实验和密度泛函理论计算,以探索催化剂的杂化纳米粒子异质结构及其催化机理。该催化剂包括限制CoO和CoP的氮掺杂碳点,并表现出强界面诱导的AB水解协同催化作用,有效降低了AB和水分子解离的能垒。N和P的共掺杂引入了许多缺陷,并进一步调节了碳层的反应性。本文介绍的多相界面设计技术为开发有效且廉价的非贵金属催化剂提供了一种新策略,该催化剂可应用于与能量催化有关的其他领域。



























 京公网安备 11010802027423号
京公网安备 11010802027423号