当前位置:
X-MOL 学术
›
Int. J. Mass Spectrom.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Protonation isomers of highly charged protein ions can be separated in FAIMS-MS
International Journal of Mass Spectrometry ( IF 1.6 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.ijms.2020.116425 J. Diana Zhang , Micah T. Donor , Amber D. Rolland , Michael G. Leeming , Huixin Wang , Adam J. Trevitt , K.M. Mohibul Kabir , James S. Prell , William A. Donald
International Journal of Mass Spectrometry ( IF 1.6 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.ijms.2020.116425 J. Diana Zhang , Micah T. Donor , Amber D. Rolland , Michael G. Leeming , Huixin Wang , Adam J. Trevitt , K.M. Mohibul Kabir , James S. Prell , William A. Donald
|
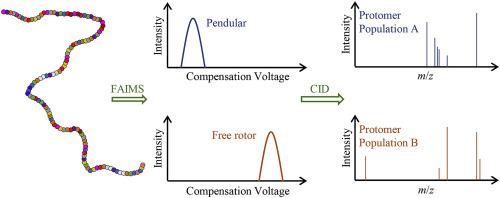
Abstract High-field asymmetric waveform ion mobility spectrometry-mass spectrometry (FAIMS-MS) can resolve over an order of magnitude more conformers for a given protein ion than alternative methods. Such an expansion in separation space results, in part, from protein ions with masses of >29 kDa undergoing dipole alignment in the high electric field of FAIMS, and the resolution of ions that adopt pendular vs free rotor states. In this study, FAIMS-MS, collision-induced dissociation (CID), and travelling wave (TW) IMS-MS were used to investigate the pendular and free rotor states of protonated carbonic anhydrase II (CAII, 29 kDa). The electrospray ionization additive 1,2-butylene carbonate was used to increase protein charge states and ensure extended ion conformations were formed. For relatively high charge states in which dipole alignment occurs (30–38+), FAIMS-MS can baseline resolve the isobaric pendular and free rotor ion populations. For TWIMS-MS, these same charge states resulted in monomodal arrival time distributions with collision cross sections corresponding to highly extended ion conformations. Interestingly, CID of FAIMS-selected pendular and free rotor ion populations resulted in significantly different fragmentation patterns. For example, CID of the dipole aligned CAII 37+ resulted in cleavages C-terminal to residue 183, 192 and 196, whereas cleavage sites for the free rotor population occurred near residues 12 and 238. Given that the cleavage sites are ’directed’ by protonation sites in the CID of protein ions, and highly charged protein ions adopt extended conformations with the same or very similar collision cross sections, these results indicate that the pendular and free rotor populations separated in FAIMS can be attributed to protonation isomers. Moreover, the extent of protein ion charging in FAIMS-MS decreased substantially as the carrier gas flow rate decreased, indicating that ion charging in FAIMS-MS can be limited by proton-transfer reactions. Given that the total mass of proton charge carriers corresponds to less than 0.2% the mass of CAII, we anticipate that FAIMS-MS can be used to separate intact isobaric proteoforms with masses of at least ∼29 kDa that result from alternative sites of post-translational modifications.
中文翻译:

可以在 FAIMS-MS 中分离高电荷蛋白质离子的质子化异构体
摘要 高场不对称波形离子迁移谱-质谱 (FAIMS-MS) 可以解析给定蛋白质离子的构象异构体比其他方法多一个数量级。分离空间的这种扩展部分是由于质量大于 29 kDa 的蛋白质离子在 FAIMS 的高电场中进行偶极排列,以及采用摆动与自由转子状态的离子分辨率。在本研究中,FAIMS-MS、碰撞诱导解离 (CID) 和行波 (TW) IMS-MS 用于研究质子化碳酸酐酶 II (CAII, 29 kDa) 的摆动和自由转子状态。电喷雾电离添加剂 1,2-碳酸亚丁酯用于增加蛋白质电荷状态并确保形成扩展的离子构象。对于偶极对齐发生的相对高电荷状态 (30–38+),FAIMS-MS 可以基线解析等压摆和自由转子离子群。对于 TWIMS-MS,这些相同的电荷状态导致单峰到达时间分布,碰撞截面对应于高度扩展的离子构象。有趣的是,FAIMS 选择的摆动和自由转子离子群的 CID 导致了显着不同的碎片模式。例如,偶极子排列的 CAII 37+ 的 CID 导致 C 端切割残基 183、192 和 196,而自由转子群的切割位点发生在残基 12 和 238 附近。鉴于切割位点是由蛋白质离子 CID 中的质子化位点,和高电荷蛋白质离子采用具有相同或非常相似的碰撞截面的扩展构象,这些结果表明在 FAIMS 中分离的摆动和自由转子群可归因于质子化异构体。此外,随着载气流速的降低,FAIMS-MS 中蛋白质离子充电的程度显着降低,表明 FAIMS-MS 中的离子充电可能受到质子转移反应的限制。鉴于质子电荷载流子的总质量对应于小于 CAII 质量的 0.2%,我们预计 FAIMS-MS 可用于分离质量至少为约 29 kDa 的完整同量异位素蛋白质型,这些蛋白质来自于后的替代位点。翻译修饰。这些结果表明在 FAIMS 中分离的摆动和自由转子群可归因于质子化异构体。此外,随着载气流速的降低,FAIMS-MS 中蛋白质离子充电的程度显着降低,表明 FAIMS-MS 中的离子充电可能受到质子转移反应的限制。鉴于质子电荷载流子的总质量对应于小于 CAII 质量的 0.2%,我们预计 FAIMS-MS 可用于分离质量至少为约 29 kDa 的完整同量异位素蛋白质型,这些蛋白质来自于后的替代位点。翻译修饰。这些结果表明在 FAIMS 中分离的摆动和自由转子群可归因于质子化异构体。此外,随着载气流速的降低,FAIMS-MS 中蛋白质离子充电的程度显着降低,表明 FAIMS-MS 中的离子充电可能受到质子转移反应的限制。鉴于质子电荷载流子的总质量对应于小于 CAII 质量的 0.2%,我们预计 FAIMS-MS 可用于分离质量至少为 29 kDa 的完整同量异位素蛋白质型,这些蛋白质来自于后的替代位点。翻译修饰。
更新日期:2020-11-01
中文翻译:

可以在 FAIMS-MS 中分离高电荷蛋白质离子的质子化异构体
摘要 高场不对称波形离子迁移谱-质谱 (FAIMS-MS) 可以解析给定蛋白质离子的构象异构体比其他方法多一个数量级。分离空间的这种扩展部分是由于质量大于 29 kDa 的蛋白质离子在 FAIMS 的高电场中进行偶极排列,以及采用摆动与自由转子状态的离子分辨率。在本研究中,FAIMS-MS、碰撞诱导解离 (CID) 和行波 (TW) IMS-MS 用于研究质子化碳酸酐酶 II (CAII, 29 kDa) 的摆动和自由转子状态。电喷雾电离添加剂 1,2-碳酸亚丁酯用于增加蛋白质电荷状态并确保形成扩展的离子构象。对于偶极对齐发生的相对高电荷状态 (30–38+),FAIMS-MS 可以基线解析等压摆和自由转子离子群。对于 TWIMS-MS,这些相同的电荷状态导致单峰到达时间分布,碰撞截面对应于高度扩展的离子构象。有趣的是,FAIMS 选择的摆动和自由转子离子群的 CID 导致了显着不同的碎片模式。例如,偶极子排列的 CAII 37+ 的 CID 导致 C 端切割残基 183、192 和 196,而自由转子群的切割位点发生在残基 12 和 238 附近。鉴于切割位点是由蛋白质离子 CID 中的质子化位点,和高电荷蛋白质离子采用具有相同或非常相似的碰撞截面的扩展构象,这些结果表明在 FAIMS 中分离的摆动和自由转子群可归因于质子化异构体。此外,随着载气流速的降低,FAIMS-MS 中蛋白质离子充电的程度显着降低,表明 FAIMS-MS 中的离子充电可能受到质子转移反应的限制。鉴于质子电荷载流子的总质量对应于小于 CAII 质量的 0.2%,我们预计 FAIMS-MS 可用于分离质量至少为约 29 kDa 的完整同量异位素蛋白质型,这些蛋白质来自于后的替代位点。翻译修饰。这些结果表明在 FAIMS 中分离的摆动和自由转子群可归因于质子化异构体。此外,随着载气流速的降低,FAIMS-MS 中蛋白质离子充电的程度显着降低,表明 FAIMS-MS 中的离子充电可能受到质子转移反应的限制。鉴于质子电荷载流子的总质量对应于小于 CAII 质量的 0.2%,我们预计 FAIMS-MS 可用于分离质量至少为约 29 kDa 的完整同量异位素蛋白质型,这些蛋白质来自于后的替代位点。翻译修饰。这些结果表明在 FAIMS 中分离的摆动和自由转子群可归因于质子化异构体。此外,随着载气流速的降低,FAIMS-MS 中蛋白质离子充电的程度显着降低,表明 FAIMS-MS 中的离子充电可能受到质子转移反应的限制。鉴于质子电荷载流子的总质量对应于小于 CAII 质量的 0.2%,我们预计 FAIMS-MS 可用于分离质量至少为 29 kDa 的完整同量异位素蛋白质型,这些蛋白质来自于后的替代位点。翻译修饰。











































 京公网安备 11010802027423号
京公网安备 11010802027423号