Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2020-09-06 , DOI: 10.1016/j.bmcl.2020.127520 Sha Ding 1 , Maryam Ghavami 1 , Joshua H Butler 2 , Emilio F Merino 2 , Carla Slebodnick 1 , Maria B Cassera 2 , Paul R Carlier 1
|
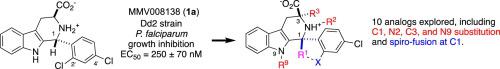
The antimalarial candidate MMV008138 (1a) is of particular interest because its target enzyme (IspD) is absent in human. To achieve higher potency, and to probe for steric demand, a series of analogs of 1a were prepared that featured methyl-substitution of the B- and C-rings, as well as ring-chain transformations. X-ray crystallography, NMR spectroscopy and calculation were used to study the effects of these modifications on the conformation of the C-ring and orientation of the D-ring. Unfortunately, all the B- and C-ring analogs explored lost in vitro antimalarial activity. The possible role of steric effects and conformational changes on target engagement are discussed.
中文翻译:

探测抗疟药四氢-β-咔啉 MMV008138 的 B 和 C 环的空间和构象约束。
抗疟候选药物 MMV008138 ( 1a ) 特别令人感兴趣,因为它的靶酶 (IspD) 在人类中不存在。为了获得更高的效力,并探测空间需求,制备了一系列1a类似物,其特征是 B 环和 C 环的甲基取代以及环链转换。X射线晶体学、核磁共振光谱和计算被用来研究这些修饰对C环构象和D环取向的影响。不幸的是,所有探索的 B 环和 C 环类似物都失去了体外抗疟活性。讨论了空间效应和构象变化对目标参与的可能作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号