当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Proton Transfer Reaction Rates in Phenol-Ammonia Cluster Cation.
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-09-03 , DOI: 10.1021/acs.jpca.0c05688 Hiroto Tachikawa 1 , Tetsuji Iyama 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-09-03 , DOI: 10.1021/acs.jpca.0c05688 Hiroto Tachikawa 1 , Tetsuji Iyama 1
Affiliation
|
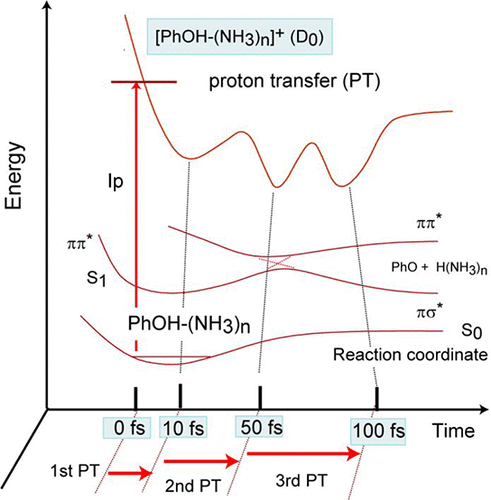
Proton transfer (PT) in an interaction system of a hydroxyl–amino group (OH–NH) plays a crucial role in photoinduced DNA and enzyme damage. A phenol–ammonia cluster is a prototype of an OH–NH interaction and is sometimes used as a DNA model. In the present study, the reaction dynamics of phenol–ammonia cluster cations, [PhOH–(NH3)n]+ (n = 1–5), following ionization of the neutral parent clusters, were investigated using a direct ab initio molecular dynamics (AIMD) method. In all clusters, PTs from PhOH+ to (NH3)n were found postionization, the reaction of which is expressed as PhOH+–(NH3)n → PhO–H+(NH3)n. The time of the PT was calculated as 43 (n = 1), 26 (n = 2), and 13 fs (n = 3–5), suggesting that the rate of PT increases with an increase in n and is saturated at n = 3–5. The difference in the PT rate originates strongly from the proton affinity of the (NH3)n cluster. In the case of n = 3–5, a second PT was found, the reaction of which is expressed as PhO–H+(NH3)n → PhO–NH3–H+(NH3)n−1, and a third PT occurred at n = 4 and 5. The time of the PT was calculated as 10–13 (first PT), 80–100 (second PT), and 150–200 fs (third PT) in the case of larger clusters (n = 4 and 5). The reaction mechanism based on the theoretical results is discussed herein.
中文翻译:

苯酚-氨团簇阳离子中的质子转移反应速率。
羟基-氨基(OH-NH)相互作用系统中的质子转移(PT)在光诱导的DNA和酶损伤中起关键作用。苯酚-氨簇是OH-NH相互作用的原型,有时被用作DNA模型。在本研究中,使用直接从头算分子动力学研究了中性母体簇电离后苯酚-氨簇阳离子[PhOH–(NH 3)n ] +(n = 1–5)的反应动力学。 (AIMD)方法。在所有簇中,发现从PhOH +到(NH 3)n的PTs发生离子化,其反应表示为PhOH + –(NH 3)n→PhO–H +(NH 3)n。在PT的时间被计算为43(ñ = 1),26(Ñ 13个FS(= 2),和Ñ = 3-5),这表明的PT增加与增加的速率Ñ和在饱和Ñ = 3–5。PT率的差异主要源于(NH 3)n簇的质子亲和力。在n = 3-5的情况下,发现了第二个PT,其反应表示为PhO–H +(NH 3)n →PhO–NH 3 –H +(NH 3)n -1,并且第三PT发生在n = 4和5处。计算得出PT的时间为10-13(第一PT),80-100(第二PT)和150-200 fs(第三PT)。较大簇的情况( n = 4和5)。本文讨论了基于理论结果的反应机理。
更新日期:2020-10-02
中文翻译:

苯酚-氨团簇阳离子中的质子转移反应速率。
羟基-氨基(OH-NH)相互作用系统中的质子转移(PT)在光诱导的DNA和酶损伤中起关键作用。苯酚-氨簇是OH-NH相互作用的原型,有时被用作DNA模型。在本研究中,使用直接从头算分子动力学研究了中性母体簇电离后苯酚-氨簇阳离子[PhOH–(NH 3)n ] +(n = 1–5)的反应动力学。 (AIMD)方法。在所有簇中,发现从PhOH +到(NH 3)n的PTs发生离子化,其反应表示为PhOH + –(NH 3)n→PhO–H +(NH 3)n。在PT的时间被计算为43(ñ = 1),26(Ñ 13个FS(= 2),和Ñ = 3-5),这表明的PT增加与增加的速率Ñ和在饱和Ñ = 3–5。PT率的差异主要源于(NH 3)n簇的质子亲和力。在n = 3-5的情况下,发现了第二个PT,其反应表示为PhO–H +(NH 3)n →PhO–NH 3 –H +(NH 3)n -1,并且第三PT发生在n = 4和5处。计算得出PT的时间为10-13(第一PT),80-100(第二PT)和150-200 fs(第三PT)。较大簇的情况( n = 4和5)。本文讨论了基于理论结果的反应机理。










































 京公网安备 11010802027423号
京公网安备 11010802027423号